ζ . Kristallogr. NCS 2 1 3 (1998) 6 6 9 - 6 7 0
669
© by R. Oldenbourg Verlag, München
C r y s t a l s t r u c t u r e o f t h e c l a t h r a t e s R b s A l g G e a s a n d R b g A l g S n s s
R. Kröner, К. Peters, H. G. von Schnering
Max-Planck-Institut für Festkörperforschung. Heisenbergstraße 1, D-70569 Stuttgart. Germany
and R. Nesper
ΕΤΗ Zürich. Laboratorium für Anorganische Chemie, Universitätsstr. 6, CH-8092 Zürich, Switzerland
Received March 3. 1998, transferred to Ist update of database ICSD in 1999, CSD-No. 409249 and CSD-No. 409250
1. R b s A l s G e a s
Source of material: Stoichiometric mixtures of the elements were sealed in Nb/Ta capsules under Ar, heated up to 1270 К (3 h), annealed at 9 7 0 К (2-3 d) and cooled down. RbgAlsGeag forms small crystals and is a semiconductor. The grey brittle substance is stable against oxygen, dilute acids and bases solutions.
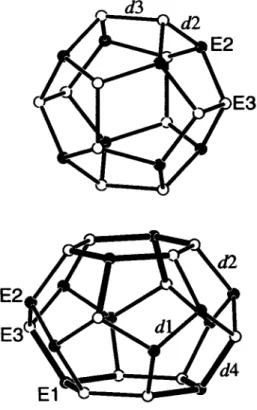
T h e refinement of the site occupancies results in the composition Rb8Al„SÌ46-n with η = 7.84 + 0.52. Bond lengths in the E46 net (figure): dl = 2.485(1) k, dl = 2.497(1) Â, <0 = 2.546(1) Â, d4
= 2.535(1) Â.
Al8Ge38Rb8, cubic, Ρηβη (No. 223), α =10.822(2) A, V = 1 2 6 7 . 4 Â ^ Z = l , R(F) =0.023, R^^F) =0.016.
Table 1. Parameters used for the X-ray data coUection
Crystal: grey, metallic luster, size 0.1 χ 0.1 χ 0.1 mm Wavelength: Mo Ka radiation (0.71069 Â)
μ: 293.2 cm"'
Diffractometer: SYNTEXPl
Scan mode: ω
Τ measurement· 293 К
2θιη3χ: 55°
ЩНк1)ипи,ие: 225 Criterion for Io. /ο>3σ(/ο) Ы(рагет)гфии: 19
Programs: SHELXS-86, SHELX-76, SHELXTL-pIus
Table 2. Final atomic coordinates and displacement parameters (in Â^)
Atom Site Occ. X У ζ Un U22 Í/33 1/12 Í/13 Í/23
Rbl 2a 0 0 0 0.0134(7) Un Un 0 0 0
Rb2 bd 1/4 1/2 0 0.0170(9) 0.0283(7) U22 0 0 0
El(Al) 6c 0.92(2) 1/4 0 1/2 0.0089(9) 0.009(1) U22 0 0 0
El(Ge) 6c 0.08 1/4 0 1/2 0.0089 0.009 U22 0 0 0
E2(A1) I61 0.04 0.1837 X χ 0.0105 Un Un -0.0003 Un Un
E2(Ge) 16/ 0.96(1) 0.1837(1) X χ 0.0105(3) Un Un -0.0003(2) Un Un
E3(A1) 24it 0.07 0 0.3067 0.1176 0.0100 0.0107 0.0097 0 0 -0.0008
E3(Ge) 24it 0.93(1) 0 0.3067(1) 0.1176(1) 0.0100(4) 0.0107(5) 0.0097(5) 0 0 -0.0008(4)
670
2. RbsAlsSnas
S o u r c e of material: For the synthesis and the chemical properties see a b o v e (RbgAIsGess)· R b g A l s S n s s is also a semiconductor. T h e grey brittle substance f o r m s well-shaped crystals with m a n y faces ( s e e r e f . 1).
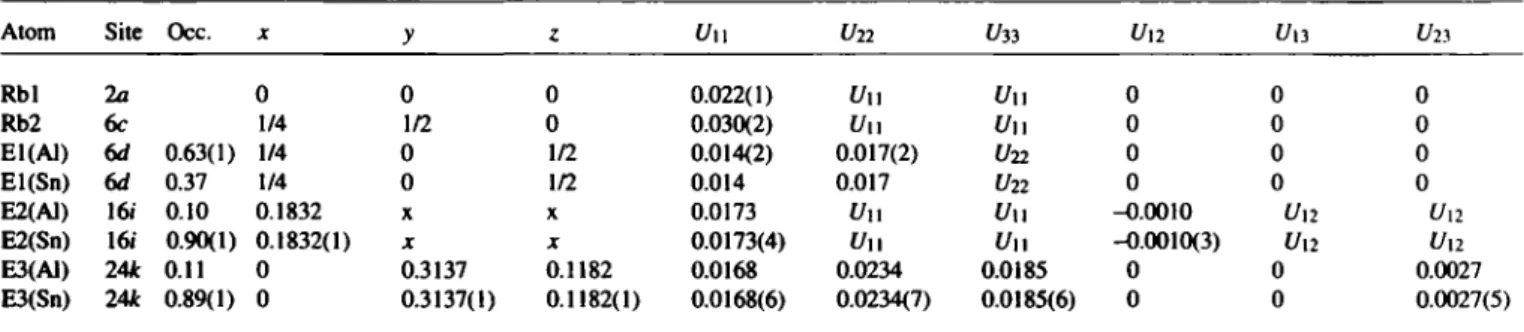
T h e r e f i n e m e n t of the site occupancies results in the composition Rb8Al/^n46-n with η = 8.02 ± 0.46. B o n d lengths in the E46 net (figure): d\ = 2.785(1) Â, íÍ2 = 2.818(1) Â, d3 = 2.845(1) Â, d4
= 2 . 7 1 7 ( 1 ) Â .
AlgRbsSnas, cubic, Ρηβη (No. 223), α =12.036(2) Λ, V = 1 7 4 3 . 6 Λ ^
Z=\,R(F) =0.049, R^F) =0.042.
Clathrates RbgAlsGesg and Rb8Al8Sn38
Table 3. Parameters used for the X-ray data collection
Crystal: grey, metallic luster, size 0.3 χ 0.4 χ 0.2 mm Wavelength: Mo Ka radiation (0.71069 Â)
μ: 179.7 cm"'
Diffractometer: SYNTEX P3
Scan mode: ω
Tmeasuremeni'- 293 К
2втах: 55°
385 Criterion for ¡o. / ο > 3 σ ( / ο )
ìi(param)refim(r· 19
Programs: SHELXS-86. SHELX-76, SHELXTL-plus
Table 4. Final atomic coordinates and displacement parameters (in Â^)
Atom Site Occ. X .y ζ Un U22 í/33 Un Uli Í/23
Rbl 2a 0 0 0 0.022(1) и и υ и 0 0 0
Rb2 6c 1/4 1/2 0 0.030(2) ί/м Un 0 0 0
El(Al) 0.63(1) 1/4 0 1/2 0.014(2) 0.017(2) Ui2 0 0 0
El(Sn) 6d 0.37 1/4 0 1/2 0.014 0.017 U22 0 0 0
E2(A1) I6i O.IO 0.1832 X X 0.0173 Un Uu -0.0010 Un Un
E2(Sn) 16i 0.90(1) 0.1832(1) X X 0.0173(4) í / l l Un -0.0010(3) Un Un
E3(A1) 24it 0.11 0 0.3137 0.1182 0.0168 0.0234 0.0185 0 0 0.0027
E3(Sn) 24jt 0.89(1) 0 0.3137(1) 0.1182(1) 0.0168(6) 0.0234(7) 0.0185(6) 0 0 0.0027(5)
References
I. Kroner, R.: Zintl-Phasen der Alkalimetalle und des Banums mit Clathrat- struknir. Dissertation, Universität Snjttgait, 1989.
3. Sheldrick, G. M.: Phase Annealing in SHELX-90: Direct Methods for Large Structures. Acta Crystallogr. KA6 (1990) 467-473.
2. Sheldrick, G. M.: SHELX-76, Programs for Crystal Strucnires Determina- tion. Cambridge 1976.
4. Sheldrick, G. M.: Program Package SHELXTL-plus. Release 4.1.
Siemens Analytical X-Ray Instruments Inc., Madison (WI53719), USA 1990.