ζ . Kristallogr. NCS 213 (1998) 4 6 3 - ^ 6 4
463
' by R. Oldenbourg Verlag, MünchenCrystal structure of dysprosium pentaphosphide, DyPs and of holmium pentaphosphide, H0P5
H. G. von Schnering, M. Wittmann and K. Peters
Max-Planck-Institut für Festkörperforschung, Heisenbergstraße 1, D-70569 Stuttgart. Germany
Received December 17, 1997, transferred to 2nd update of database ICSD in 1998, CSD-No. 409184 and CSD-No. 409185
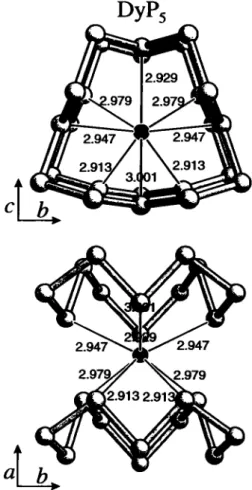
1. Dysprosium pentaphosphide, DyPs
Source of material: The compound was synthesized by reaction of the elements in a sealed glass ampoule at 813 К (2 weeks) in the presence of molten CsCl/KJ and small amounts of iodine (see ref. 1). DyPs forms bright black crystals of prismatic or columnar shape, which are stable against dilute bases and non-oxidizing acids. DyPs is paramagnetic with pefr= 10.5 ць (see ref. 2).
DyPs crystallizes in the space group /^i/m and is isotypic to NdPs (see ref. 3). The P-P distances vary between 2.160 Â and 2.215 Â, the Dy-P distances vary between 2.913 Â and 3.001 Â.
DyPs, monoclinic, P12i/ml (No. 11), a =4.901(2) Â, b =9.396(3) Â, с =5.338(3) Â, β =102.53(4)°, V =240.0 Â^ Ζ =2, ÄfFJ =0.036, Ry^F) =0.039.
Table 1. Parameters used for the X-ray data collection
Crystal: black, spherical, metallic luster, size 0.1 mm Wavelength: Mo Ka radiation (0.71073 Â)
μ: 170.4 cm"'
Difíractometer: SYNTEXPI
Scan mode: ω
Tmeasuremeni·
293 К20niax: 55°
ЩНк1)шш,ш··
581 Criterion for lo'· /ο>2σ(/ο)Îi(param)r<imed·.
31Program: SHELXTL-plus
Table 2. Final atomic coordinates and displacement parameters (in Â^)
Atom Site
X У
ζUn Un иъъ
{/12Un
t/23Dy
2e
0.0070(1) 1/4 0.3503(1) 0.0204(3) 0.0171(3) 0.0277(4) 0 0.0063(3) 0P(l) 4/ 0.3814(5) 0.5920(3) 0.0421(5) 0.020(1) 0.017(1) 0.028(1) 0.0063(9) 0.0001(9) 0.0000(9) P(2) 4/ 0.2846(5) 0.5300(3) 0.4026(5) 0.021(1) 0.018(1) 0.030(1) 0.0004<9) 0.0095(9) 0.0003(9)
P(3)
2e
0.2769(7) 1/4 0.8966(7) 0.021(2) 0.019(2) 0.028(2) 0 0.007(1) 0464
DyPs and H0P5HoP,
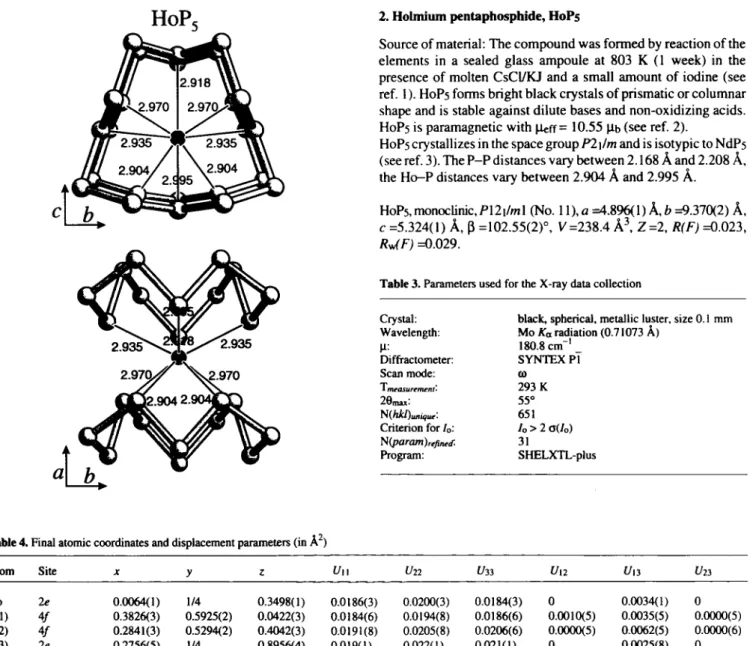
2. Holmium pentaphosphide, H0P5Source of material: The c o m p o u n d w a s formed by reaction o f the elements in a sealed glass ampoule at 8 0 3 К (1 w e e k ) in the presence of molten CsCl/KJ and a small amount of iodine (see ref. 1 ). H0F5 forms bright black crystals o f prismatic or columnar shape and is stable against dilute bases and non-oxidizing acids.
H0P5 is paramagnetic with ЦеГГ = 10.55 Ць (see ref. 2).
H0P5 crystallizes in the space group F 2 | / / n and is isotypic to N d P s (see ref. 3). The P - P distances vary b e t w e e n 2 . 1 6 8 Â and 2 . 2 0 8 Â, the H o - P distances vary between 2 . 9 0 4 Â and 2.995 Â.
HoP5,monocUnic,P12i/ml (No. 11), a =4.896(1 )Â,i> =9.370(2) Â, с = 5 . 3 2 4 ( 1 ) Â, β = 1 0 2 . 5 5 ( 2 ) ° , V = 2 3 8 . 4 Â ^ Z = 2 , R ( F ) =^.02Ъ,
R^^F) = 0 . 0 2 9 .
Table 3. Parameters used for the X-ray data collection
Crystal: black, spherical, metallic luster, size 0.1 mm Wavelength: Mo Ka radiation (0.71073 À)
μ: 180.8 cm"'
Diffractometer: SYNTEX PI
Scan mode: ω
T/wasuremenl' 293 К
2втах: 55°
651 Criterion for ¡0: / ο > 2 σ ( / ο )
N{param)refineif· 31
Program: SHELXTL-plus
Table 4. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X У ζ ί/ιι U22 Uìì ί/12 Un игъ
Ho 2e 0.0064(1) 1/4 0.3498(1) 0.0186(3) 0.0200(3) 0.0184(3) 0 0.0034(1) 0
P(l) 4 / 0.3826(3) 0.5925(2) 0.0422(3) 0.0184(6) 0.0194(8) 0.0186(6) 0.0010(5) 0.0035(5) 0.0000(5) P(2) 4 / 0.2841(3) 0.5294(2) 0.4042(3) 0.0191(8) 0.0205(8) 0.0206(6) 0.0000(5) 0.0062(5) 0.0000(6)
P(3) 2e 0.2756(5) 1/4 0.8956(4) 0.019(1) 0.022(1) 0.021(1) 0 0.0025(8) 0
References
1. Wittmaon, M.: DarsteUung, Struktur und Eigenschaften von Polyphos- phiden und Polyaiseniden der Seltenerdmetalle. Dissertation, Universität Münster, Germany 1977.
2. Hartweg, M. : Über physikalische Eigenschaften der Seltenerd-Pentaphos- phide LnPs, von СеРг und LaAs2, sowie zur Kenntnis einiger neuer temärer Alkalimetall-Europium-Pnictide. Dissertation, Universität Stutt- gart, Germany 1987.
Wichelhaus, W.; von Schnering, H. G.: Die Pentaphosphide des Lanthans und Neodyms, LaPs und NdPs. Z. Anorg. Allg. Chem. 419 (1976) 77-86.
Sheldrick, G. M.: SHELXTL, an integrated system for solving, refining and displaying crystal structures from diffraction data. University of Göttingen, Germany 1978.