ζ. Kristallogr. NCS 213 (1998) 459
© by R. Oldenbourg Verlag, München
Crystal structure of yttrium pentaphosphide, YP5
H. G. von Schnering, D. Vu and K. Peters
Max-Planck-Inslitut für Festkörperforschung, Heisenbergstraße 1, D-70569 Stuttgart, Germany
Received December 17. 1997, transferred to 2nd update of database ICSD in 1998, CSD-No. 409188
Source of material: The compound was synthesized from filings of Y metal and red Ρ (molar ratio 1:7) with small amounts of iodine in presence of molten LiCl/KJ in a sealed glass ampoule at 793 К (2 weeks, see refs. 1-4). The black prismatic crystals of YPs are stable against dilute HCl and KOH. Thermogravimetrical investigations show that YP5 decomposes at 748 К into VP and P.
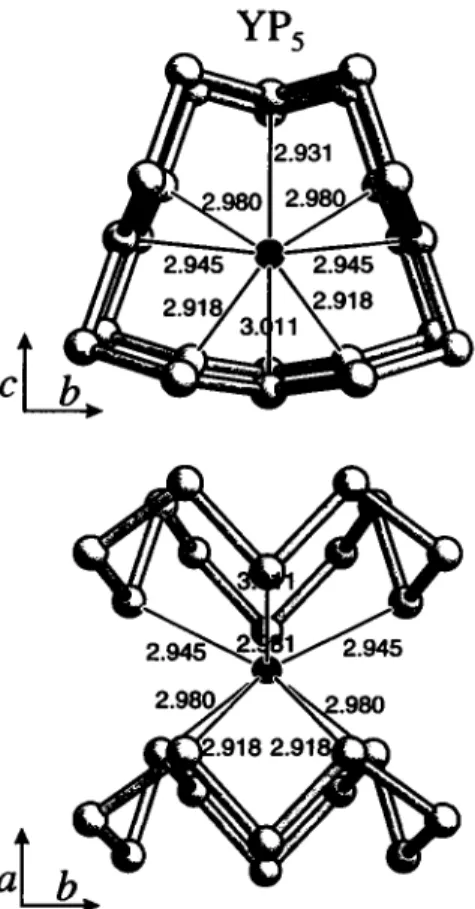
YP5 crystallizes in the space group P2i/m isotypically to NdPs.
The bond lengths vary between 2.165 Â and 2.207 Л for the P-P bonds and 2.918 Â and 3.011 Â for the Y-P bonds.
P5Y, monoclinic, P\2\lm\ (No. 11), a =4.911(1) Â, b =9.384<2) Â, с =5.344(1) Â, β =102.50(2)°, V =240.4 Â^ Ζ =2, ÄfF) =0.023, Rv^F) =0.023.
Table 1. Parameters used for the X-ray data collection
Crystal: black prisms, size 0.01 χ 0.02 χ 0.02 mm Wavelength: Mo Ka radiation (0.71073 À)
μ: 136.0 cm"'
Difftactometer: S Y N T E X P I
Scan mode: ω
T ^ i o i u « ™ « , : 293 К
20max: 55°
ЩИкОшщие: 645
Criterion for /о: / ο > 2 σ ( / ο )
^(рагат)гфи<1: 31
Program: SHELXTL-plüs
Table 2. Final atomic coordinates and displacement parameters (in A^)
Atom Site X y ζ í/ll U22 Un Un Í/13 Í/23
Y 2e 0.0064(1) 1/4 0.3497(1) 0.0105(2) 0.0083(2) 0.0091(2) 0 0.0017(2) 0
P(l) 4 / 0.3825(2) 0.5920(1) 0.0431(2) 0.0113(4) 0.0094(4) 0.0106(4) 0.0005(3) 0.0019(3) 0.0005(3) P(2) 4 / 0.2853(2) 0.5296(1) 0.4039(2) 0.0124(4) 0.0112(4) 0.0123(4) -0.0002(3) 0.0045(3) 0.0000(3)
P(3) le O.miO) 1/4 0.8952(3) 0.0121(6) 0.0102(6) 0.0131(6) 0 0.0028(5) 0
References
1. Wichelhaus, W.; von Schnering, H. G.: Die Pentaphosphide des Lanthans und Neodyms, LaPs und NdPs. Z. Anorg. Allg. Chem. 419 (1976) 77-86.
2. Menge, G.; von Schnering, Η. G.: Gadoliniumpentaphosphid GdPs. Ζ.
Anorg. Allg. Chem. 422 (1976) 226-230.
3. Wittmann, M.: Darstellung, Struktur und Eigenschaften von Polyphos- phiden und Polyarseniden der Seltenerdmetalle. Dissertation, Universität Münster, Germany 1977.
von Schnering, H. G.; Wichelhaus, W.; Wittmann, M.: New Polyphos- phides of the Rare Earth Metals. Abstracts Vth Intem. Conf. Solid Comp, of Transition Elements (ICSTE) Uppsala, Sweden 1976.
Sheldrick, G. M.: SHELXTL, an integrated system for solving, refining and displaying crystal structures from diffiractìon data. University of Göttingen, Germany 1981.
4.
5.