Ζ. Kristallogr. NCS 214 (1999) 17-18
© by R. Oldenbourg Verlag, München
17
Crystal structure of tetrarubidium cyclo -hexaarsenide(4-), Rb4As6 and of tetracesium cycfo-hexaarsenide(4-), CS4AS6
W. Hönle, G. Krogull, Κ. Peters and H. G. von Schnering
Max-Planck-Institut für Festkörperforschung, Heisenbergstraße 1, D-70569 Stuttgart, Germany
Received August 26, 1998, transferred to 1st update of database ICSD in 1999, CSD-No. 409381 and CSD-No. 409382
Source of material
Quartz ampoules with an inner quartz tube were loaded under inert conditions and cooling (CH3OH/CO2; caution!) with the elements in stoichiometric ratios and closed. The ampoules were slowly heated (1-2 d) up to 750 Κ - 800 Κ in a tilted furnace, annealead for one week and slowly cooled down to room tempe- rature (12 h). Both compounds form blue reflecting black crystals with large faces and they are very sensitive to oxidation and hydrolysis [1],
Discussion
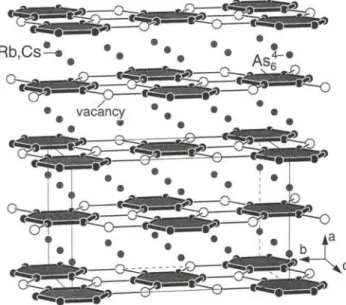
Both compounds are isotypic with OC-K4P6. A complete descrip- tion of the structure type is given in [2-5], For the chemical bonding in the ΙΟπ-Hückel arene [Asé] see [5, 6]. The bond lengths in the almost regular 6-membered rings are d(As-As) = 2.380(3) Â (Rb) and 2.371(3) Â (Cs). The Rb-As and Cs-As distances vary in the range 3.591 Â - 3.684 Â and 3.718 Â - 3.854 Â, respectively.
1. Tetrarubidium cyc/o-hexaarsenide, RbiAse Abstract
As6Rb4, orthorhombic, Fmmm (No. 69), a = 10.078(2) Â, b = 15.186(3) Â, c = 9.170(2) Â, V= 1403.4 Â
3, Z = 4, Rg{F) = 0.024, R
W(F) = 0.026, T= 293 K.
As6Cs4, orthorhombic, Fmmm (No. 69), a = 10.373(2) Â, b = 15.577(3) Â, c = 9.764(2) Â, V= 1577.7 Â
3, Z = 4, tfgtfFJ = 0.059, Rw(F) = 0.056, T= 293 K.
Table 1. Data collection and handling.
Crystal:
Wavelength:
μ:
Diffractometer, scan mode:
20']1L1.\ •
W¡i/)measured,
N(hkl)unique:
Criterion for Fobs,
N(hkl) t i.
N(param) refined:
Program:
steel-blue platelet, size 0.2 χ 0.2 χ 0.1 mm Ag Ka radiation (0.56087 Â)
148.4 cm"1 _ SYNTEX PI, ω 40°
152, 152
f o b s > 3 a(Fobs), 152 19
SHELXTL-plus [7]
Table 2. Atomic coordinates and displacement parameters (in Â2).
Atom Site
X y ζ Un U22
C/33Un
C/13 C/23Rb(l) 8« 0.2328(3) 1/2 0 0.030(2) 0.044(2) 0.035(3) 0 0 0
Rb(2) 8/ 1/4
1/4
1/4 0.040(2) 0.035(1) 0.049(3) 0 0 0As(l) 8A 1/2 0.6576(2) 0 0.039(2) 0.033(2) 0.032(2) 0 0 0
As(2) 16m 0 0.5782(2) -0.2760(3) 0.038(1) 0.034(1) 0.032(1) 0 0 -0.002(1)
vacancy
18
R b 4 A s 6 a n d C s 4 A s 62 . T e t r a c e s i u m c y c / o - h e x a a r s e n i d e , CS4AS6
Table 3. Data collection and handling.
Crystal:
Wavelength:
μ:
Diffractometer, scan mode:
20max:
NfA/c/jmeasured, WtWjunique:
Criterion for Fobs:
N(param) refined:
Program:
steel-blue platelet, size 0 . 1 5 x 0 . 2 x 0 . 0 8 m m M o Ko. radiation (0.71073 Â) 216.1 cm"1
Siemens-Nicolet, ω 6 0 °
5 4 9 , 5 4 9
Fobs > 3 a f F o b d 547 19
SHELXTL-plus [7]
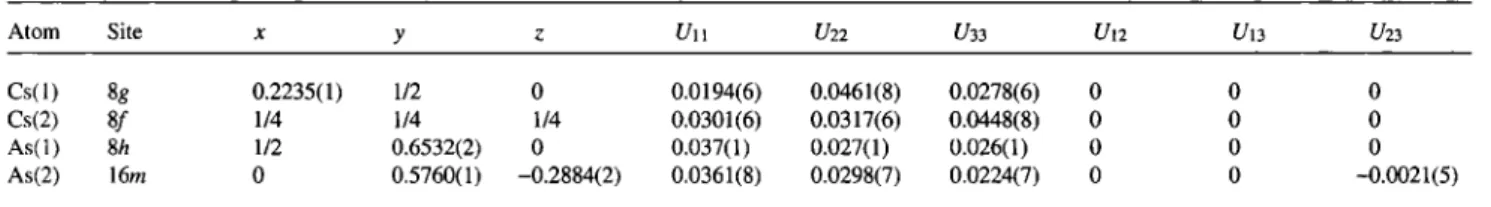
Table 4. Atomic coordinates and displacement parameters (in Â2).
Atom Site X y ζ Un C/22 C/33 C/12 C/13 C/23
C s ( l ) 8« 0.2235(1) 1/2 0 0.0194(6) 0.0461(8) 0.0278(6) 0 0 0
Cs(2) 8 / 1/4 1/4 1/4 0.0301(6) 0.0317(6) 0.0448(8) 0 0 0
A s ( l ) 8A 1/2 0.6532(2) 0 0.037(1) 0.027(1) 0.026(1) 0 0 0
As(2) 16m 0 0.5760(1) - 0 . 2 8 8 4 ( 2 ) 0.0361(8) 0.0298(7) 0.0224(7) 0 0 -0.0021(5)
R e f e r e n c e s
1. Hönle, W.: Über niedere Phosphide, Arsenide und Antimonide der Alka- limetalle. Dissertation, Universität Münster, 1975.
2. Schmettow, W.; Lipka, Α.; von Schnering, H. G.: Rb4P6, ein Phosphidmit planaren Phosphorsechsringen. Angew. Chem. 86 (1974) 379-380; An- gew. Chem. Int. Ed. Engl. 13 (1974) 345-346.
3. Abicht, H. P.; Hönle, W.; von Schnering, H. G.: Tetrakalium hexaphos- phid: Darstellung, Struktur und Eigenschaften von OÍ-K4P6 und ß-IQPö.
Z. Anorg. Allg. Chem. 519 (1984) 7-23.
4. von Schnering, H. G.: Homonucleare Bindungen bei Hauptgruppenele- menten. Angew. Chem. 93 (1981) 44-63; Angew. Chem. Int. Ed. Engl. 2 0 (1981)33-51.
5. von Schnering, H. G.; Hönle, W.; Schmettow, W.; Hinze, U.; Bauhofer, W.; Kliche, G.: Tetrarubidiumhexaphosphid und Tetracäciumhexaphos- phid: Darstellung, Struktur und Eigenschaften von Rb4P6 und CS4P6· Z.
Anorg. Allg. Chem. 553 (1987) 261-279.
6. von Schnering, H. G.; Bolle, U.; Curda, J.; Peters, K.; Carrillo-Cabrera, W.; Somer, M.; Schultheiss, M.; Wedig, U.: Hückel-Arene mit zehn Tt-Elektronen: Die cyclischen Zintl-Anionen S ¡ 6 u n d G e s '0 - isoster mit P64" und Ase4". Angew. Chem. 108(1996) 1062-1064; A n g e w . C h e m . Int.
Ed. Engl. 35 (1996) 984-986.
7. Sheldrick, G. M.: Program Package SHELXTL-plus. Release 4.1. Sie- mens Analytical X-Ray Instruments Inc., Madison (WI 53719), U S A 1990.