ζ . Kristallogr. NCS 213 (1998) 665-666
665
© by R. Oldenbourg Verlag, München
C r y s t a l s t r u c t u r e o f t h e n o v e l c h i r a l c l a t h r a t e , B a 6 l i i 4 G e 2 i
H. G. von Schnering, R. Kröner, W. Carrillo-Cabrera, К. Peters
Max.-Planck-Institut für Festkörperforschung, Heisenbergstraße 1, D-70569 Stuttgart, Germany
and R. Nesper
EHT Zürich. Laboratorium für Anorganische Chemie, Universitätsstraße 6, CH-8092 Zürich, Switzerland Received March 3, 1998, transferred to Ist update of database ICSD in 1999, CSD-No. 409266
Source of material: The compound was synthesized from the elements under Ar in a sealed Nb capsule (ratio 4:6:21; heating up to 1320 K; cooling down (1 d)). The silvery grey, brittle compound is a semiconductor, and stable against air, moisture and dilute acids and bases. The chemical analysis with selected crystals gave Ba6.2ln4.3Ge20.7(AES-ICP) (see ref. 1).
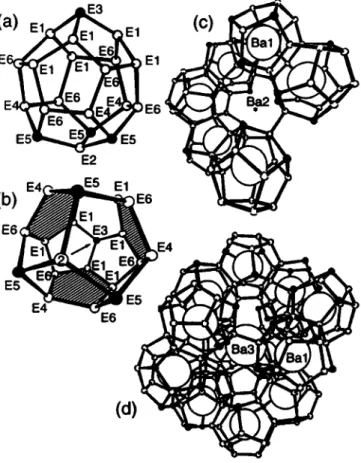
Ваб1п4Се21 forms a novel chiral structure (cPlTA, see refs. 1, 2) which was also found with other compounds in the last years (see refs. 3, 4). The refinement of the occupancies gave the composi- tion Ваб1плСе25-л with η = 4.06 ± 0.13 . The structure is charac- terized by a network of condensed pentagondodecahedra (pdod).
Each pdod shares faces (shaded in figure b) with three others and is connected to a fourth via an additional skelton bond (figure c).
The essential pdod (figure a) consists of 4 threefold (black) and 16 fourfold bonded atoms, leading to 108 bonding electrons per formular unit: [In4Ge2i] Each pdod contains the complete information about the chirality (chains E3-E1-E1, see figure b).
Fragments of the network are (4,4,4)-barrelans (figure d) as well as (4,4,4)-propeUans (one wing see figure c) formed by condensed pdods. Hie general pattern of the cP124 structure is described by i(MM'2M"3[(3b)Y8(4b)Xi7]), M = Баз, M' = Bal, Μ" = Β ώ , with 8 Ge atoms at the threefold bonded (3b)Y nodes (as Ge") and with 17 E atoms at the fourfold bonded (4b)X nodes (as In~, Ge®). The structure is a hierachical derivative of β-Μη and AI2CM03 (Bal, ВаЗ, Ва2) and contains elements of clathrates and zeolites. Bal center the pdod, Ba2 and ВаЗ are in the larger and smaller open cavities of the channels. I b e anisotropic displace- ments (or split positions) of Ba2 and ВаЗ extend along the chan- nels, which follow the path of the i i i ~ У graph. The bond lengths in the Eioonet vary between 2.526 A and 2.654 Â.
BaéGeaibM, cubic, P4i32 (No. 213), a =14.739(2) Â, V =3201.9 Â^ Ζ =4, R(F) =0.032, R^Ml^) =0.065.
Table 1. Parameters used for the X-ray data collection Crystal: silvery grey polyhedron.
size 0.3 X 0.5 X 0.2 mm Wavelength: Mo Ka radiation (0.71069 A)
μ: 283.7 cm"'
Diñiactometer: SYNTEXPl
Scan mode: ω
Tm^asurr^n,: 293 К
2θιιΐ3χ: 55°
N(AW)um,„: 786
Criterion for /0: /ο>2σ(/ο) Ν(ραΓαιη)Γφκά: 57
Program: SHELXS-86, SHELXL-93
6 6 6 Ваб1п40е21
ТаЫс 2. Final atomic coordinates and displacement parameters (in Â^)
Atom Site Occ. X У ζ Un Uli t/33 Un Un U2}.
B a d ) 8c 0.05794(4) X χ 0.0139(2) Un Un 0.0002(2) U12 Un
Ba(2)'' 24i 0.5 0.1411(2) 0.1872(4) 0.4355(4) 0.060(3) 0.025(1) 0.023(1) -0.002(2) -0.008(2) 0.0063(5)
Ba(3) 4a 3/8 3/8 3/8 0.0262(3) Un Un 0.0083(4) Ur. i^i:
El(ln) 24e 0.42(1) 0.29185(5) 0.95412(6) 0.74709(5) 0.0149(4) 0.0175(4) 0.0162(4) 0.0003(3) 0.0013(3) -0.0025(3)
El(Ge) 24e 0.58 0.29185 0.95412 0.74709 0.0149 0.0175 0.0162 0.0003 0.0013 -0.0025
E2(In) 8c 0.29(2) 0.92465(6) X χ 0.0171(3) Un Un -0.0015(3) Un Un
E2(Ge) 8c 0.71 0.92465 X χ 0.0171 Un Un -0.0015(3) Ux2 Un
E3(Ge) 8c 0.21782(8) X χ 0.0255(4) Un Un 0.0019(5)
u,2
UnE4(ln) I2d 0.32(1) 1/8 0.82643(6) .ν+1/4 0.0145(5) 0.01192(4) Ul2 0.0016(3) -U\2 0.0077(5)
E4<Ge) \2d 0.68 1/8 0.82643 >•+1/4 0.0145 0.01192 Ul2 0.0016 -Un 0.0077
E5(Ge) 24e 0.91218(7) 0.08472(7) 0.85403(7) 0.0236(5) 0.0244(5) 0.0145(5) 0.0056(5) -0.0022(4) -0.0010(4) E6(Ge) 24e 0.18158(7) 0.98991(6) 0.87370(6) 0.0158(5) 0.0126(4) 0.0129(4) -0.0004(4) 0.0024(4) 0.0012(4)
Ba(2)'' \2d 1/8 0.18608(6) У+1/4 0.130(1) 0.0239(3) U22 -0.0007(5) -Un 0.0060(5)
a: Alternative Ba(2) positions.
References
Kröner, R.; Nesper, R.; von Schnering, H. G.: Ваб1п4Се21 - ein neuer QaUirat-Typ. Ζ. Kiistallogr. 182 (1988) 164.
Kröner, R.: Zintl-Phasen der Alkalimetalle und des Bariums mit Clathiat- struktur. Dissertation, Universität Stuttgart, 1989.
Zhao, J.-T.; Coibett, J. D.: Zintl Phases in Alkali-Metal-Tin Systems:
K«Sn2S with Condensed Pentagonal Dodecahedra of Tin. Two A«Sn44 Phases with a Defect Qathrate Structure. Inorg. Chem. 33 (1994) 5721- 5726.
6.
Fässler, T. F.; Kronseder, С.: K6Sn23BÌ2 und Кб5п25 - zwei Phasen mit duraler Clathrate-Stiucktur und ihr Verhalten gegenüber Ethylendiamin.
Z. Anorg. Allg. Chem. 624 (1998) 561-568.
Sheldrick, G. M.: Phase Annealing in SHELX-90: Direct Methods for Large Structures. AcU Crystallogr. A46 (1990) 467^73.
Sheldrick, G. M.: SHELXL-93. Program for refining crystal structures.
University of Göttingen, Germany 1993.