Zeitschrift für Kristallographie - New Crystal Structures 213,289-290 289
© by R. Oldenbourg Verlag, München 1998
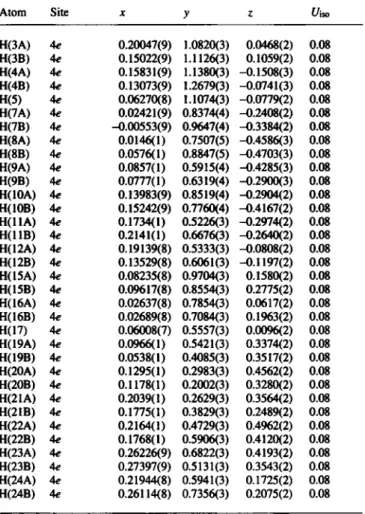
Crystal structure of (2/?,2'5,5Ä,5'5)-2,2'-bi-(2,5-epoxy-cyclododecan-l,6- dione), [Ci2Hi703]2
к . Peters, E.-M. Peters
Max-Planck-lnstitut für Festkörperforschung, Heisenbergstraße 1, D-70506 Stuttgart, Germany
M. Liebenau and W. Tochtermann
Christian-Albrechts-Universität Kiel, Institut für Organische Chemie, Olshausenstraße 40, D-24098 Kiel, Gemiany
Received August 14, 1997, CSD-No. 409071
C 2 0
i C 2 3
Source of material: The title compound was prepared by ozono- lysis of a m«5Ci-bioxanorbomene as described in ref. 1.
C24H34O6, monoclinic, Ρ12ι/α1 (No. 14), a =26.131(7) Â, b =7.947(2) Â. с =10.734(3) Â, β =99.44(2)°, V=2198.9Â^ Z=4, R(F) =0.054, Rv^F) =0.052.
Table 1. Parameters used for the X-ray data collection
Crystal: colorless column, size 0.3 χ 0.9 χ 0.25 m m Wavelength: Mo Ka radiation (0.71073 A)
μ:
0.90 c m " 'Diffractometer: Siemens R3m/V
Scan mode: Wyckoff
'^measurement'· 293 К
26max: 55°
ЩНк1)ипи,иг: 5051
Criterion for Fo. F „ > 3 o ( F o )
N(param)r^ned: n i
Program: SHELXTL-plus
T a b l e 3. Final atomic coordinates and displacement parameters (in Â^)
Table 2. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X У ζ í/iso
H(3A) 4 i 0.20047(9) 1.0820(3) 0.0468(2) 0.08 H(3B) 4e 0.15022(9) 1.1126(3) 0.1059(2) 0.08 H(4A) 4e 0.15831(9) 1.1380(3) -0.1508(3) 0.08 H(4B) 4e 0.13073(9) 1.2679(3) -0.0741(3) 0.08 H(5) 4e 0.06270(8) 1.1074(3) -0.0779(2) 0.08 H(7A) 4e 0.02421(9) 0.8374(4) -0.2408(2) 0.08 H(7B) 4e -0.00553(9) 0.9647(4) -0.3384(2) 0.08 H(8A) 4e 0.0146(1) 0.7507(5) -0.4586(3) 0.08 H(8B) 4e 0.0576(1) 0.8847(5) -0.4703(3) 0.08 H(9A) 4e 0.0857(1) 0.5915(4) -0.4285(3) 0.08 H(9B) 4e 0.0777(1) 0.6319(4) -0.2900(3) 0.08 H(IOA) 4e 0.13983(9) 0.8519(4) -0.2904(2) 0.08 H(IOB) 4e 0.15242(9) 0.7760(4) -0.4167(2) 0.08 H ( l l A ) 4e 0.1734(1) 0.5226(3) -0.2974(2) 0.08 H ( l l B ) 4e 0.2141(1) 0.6676(3) -0.2640(2) 0.08 H(12A) 4e 0.19139(8) 0.5333(3) -0.0808(2) 0.08 H(12B) 4e 0.13529(8) 0.6061(3) -0.1197(2) 0.08 H(15A) 4e 0.08235(8) 0.9704(3) 0.1580(2) 0.08 H(15B) 4e 0.09617(8) 0.8554(3) 0.2775(2) 0.08 H(16A) 4e 0.02637(8) 0.7854(3) 0.0617(2) 0.08 H(16B) 4e 0.02689(8) 0.7084(3) 0.1963(2) 0.08 H(17) 4e 0.06008(7) 0.5557(3) 0.0096(2) 0.08 H(19A) 4e 0.0966(1) 0.5421(3) 0.3374(2) 0.08 H(19B) 4e 0.0538(1) 0.4085(3) 0.3517(2) 0.08 H(20A) 4e 0.1295(1) 0.2983(3) 0.4562(2) 0.08 H(20B) 4e 0.1178(1) 0.2002(3) 0.3280(2) 0.08 H(21A) 4e 0.2039(1) 0.2629(3) 0.3564(2) 0.08 H(21B) 4e 0.1775(1) 0.3829(3) 0.2489(2) 0.08 H(22A) 4e 0.2164(1) 0.4729(3) 0.4962(2) 0.08 H(22B) 4e 0.1768(1) 0.5906(3) 0.4120(2) 0.08 H(23A) 4e 0.26226(9) 0.6822(3) 0.4193(2) 0.08 H(23B) 4e 0.27397(9) 0.5131(3) 0.3543(2) 0.08 H(24A) 4e 0.21944(8) 0.5941(3) 0.1725(2) 0.08 H(2*B) 4e 0.26114(8) 0.7356(3) 0.2075(2) 0.08
Atom Site X У г Un Un иъъ Un í/13 í/23
C ( l ) 4e 0.18772(7) 0.7853(3) -0.0526(2) 0.041(1) 0.045(1) 0.051(1) 0.0036(9) 0.0094(8) 0.0060(9) 0 ( 1 ) 4e 0.23103(5) 0.8398(2) -0.0478(2) 0.0420(8) 0.070(1) 0.090(1) -0.0047(7) 0.0226(8) -0.0081(9) C(2) 4e 0.14827(7) 0.8830(2) 0.0141(2) 0.0346(9) 0.036(1) 0.054(1) 0.0012(8) 0.0060(8) -0.0016(9) C(3) 4e 0.16350(9) 1.0677(3) 0.0345(2) 0.053(1) 0.040(1) 0.079(2) -0.003(1) 0.015(1) -0.003(1)
290
[ C l 2 H l 7 0 3 ] 2 Table 3. (Continued)Atom Site X У Τ t / | l Uli U}j и 12 Un t / 2 3
C(4) 4e 0.13742(9) 1.1506(3) -0.0857(3) 0.074(2) 0.044(1) 0.090(2) 0.003(1) 0.028(1) 0 0 1 1 ( 1 ) C(5) 4e 0.08803(8) 1.0520(3) -0.1189(2) 0.054(1) 0.051(1) 0.066(1) 0.018(1) 0.020(1) 0 0 1 6 ( 1 ) C(6) 4e 0.0644(1) 1.0507(4) -0.2582(3) 0.062(2) 0.081(2) 0.073(2) 0.020(1) 0.018(1) 0.030(1) 0 ( 6 ) 4e 0.07349(9) 1.1659(3) -0.3244(2) 0.134(2) 0.107(2) 0.0%(2) 0.003(1) 0.011(1) 0.057(1) C(7) 4e 0.02749(9) 0.9136(4) -0.3085(2) 0.055(1) 0.123(3) 0.061(2) 0.008(2) 0.006(1) 0.025(2) C(8) 4e 0.0442(1) 0.8089(5) -0.4138(3) 0.075(2) 0.138(3) 0.053(1) -0.008(2) -0.005(1) 0 0 1 6 ( 2 ) C(9) 4e 0.0858(1) 0.6790(4) -0.3668(3) 0.087(2) 0.098(2) 0.057(1) -0.007(2) 0.003(1) - O O l O d ) C(IO) 4e 0.14088(9) 0.7505(4) -0.3385(2) 0.074(2) 0.087(2) 0.046(1) 0.001(1) 0.023(1) -0.001(1) C ( l l ) 4e 0.1792(1) 0.6326(3) -0.2607(2) 0.067(1) 0.073(2) 0.059(1) 0.008(1) 0.024(1) - 0 0 1 0 ( 1 ) C(12) 4e 0.17141(8) 0.6247(3) -0.1222(2) 0.056(1) 0.046(1) 0.054(1) 0.005(1) 0013(1) -0.001(1) 0 ( 1 3 ) 4e 0.09989(5) 0.8831(2) -0.0749(1) 0.0392(7) 0.0459(8) 0.0553(8) 0.0017(6) 0.0047(6) 0.0092(7) C(14) 4e 0.13901(7) 0.7934(2) 0.1368(2) 0.039(1) 0.036(1) 0.049(1) 0.0038(8) 0.0075(8) -0.0047(8) C(I5) 4e 0.09064(8) 0.8578(3) 0.1868(2) 0.051(1) 0.047(1) 0.057(1) 0.008(1) OOI7(I) -0.003(1) C(16) 4e 0.04784(8) 0.7367(3) 0.1338(2) 0.042(1) 0.066(1) 0.068(1) 0.007(1) 0 Ό Ι 5 ( Ι ) 0.002(1) C(I7) 4e 0.07531(7) 0.5795(3) 0.0954(2) 0.040(1) 0.055(1) 0.050(1) -0.0045(9) 0.0053(8) -0.006(1) C(18) 4e 0.06907(8) 0.4231(3) 0.1726(2) 0.046(1) 0.051(1) 0.062(1) -0.008(1) 0 0 1 9 ( 1 ) - 0 0 1 0 ( 1 ) 0 ( 1 8 ) 4e 0.05230(7) 0.2958(2) 0.1206(2) 0.077(1) 0.057(1) 0.083(1) -0.0198(9) 00164(9) - 0 0 1 4 3 ( 9 ) C(I9) 4e 0.0840(1) 0.4311(3) 0.3142(2) 0.084(2) 0.064(2) 0.055(1) -0.007(1) 0.028(1) -0.003(1) C(20) 4e 0.1273(1) 0.3073(3) 0.3663(2) 0.124(2) 0.054(1) 0.049(1) 0.001(2) 0 0 1 5 ( 1 ) 0.004(1) C(2I) 4e 0.1807(1) 0.3566(3) 0.3371(2) 0.093(2) 0.050(1) 0.049(1) 0.016(1) 0.006(1) -0.000(1) C(22) 4e 0.2039(1) 0.5092(3) 0.4114(2) 0.097(2) 0.062(2) 0.043(1) 0.017(1) -0.004(1) -0.005(1) C(23) 4e 0.24792(9) 0.5%2(3) 0.3611(2) 0.065(1) 0.071(2) 0.066(1) 0.017(1) - 0 0 1 7 ( 1 ) -OOI3(1) C(24) 4e 0.23185(8) 0.6801(3) 0.2328(2) 0.042(1) 0.064(1) 0.063(1) 0.007(1) 0.001(1) -0.006(1) C(25) 4c 0.18916(8) 0.8064(3) 0.2355(2) 0.048(1) 0.046(1) 0.054(1) 0.001(1) 0.0025(9) -0.004(1) 0 ( 2 5 ) 4e 0.19309(7) 0.9175(2) 0.3126(2) 0.081(1) 0.061(1) 0.081(1) 0.0130(9) - 0 0 1 8 2 ( 9 ) -0.027(1) 0 ( 2 6 ) 4e 0.12972(5) 0.6170(2) 0.1154(1) 0.0379(7) 0.0381(7) 0.0515(8) 0.0009(6) 00102(6) -0.0024(6)
References
1. Liebenau, M.: Überbrückte funktionalisieite Tetrahydrofurane und 2,2'- verknüpfte Bitetrahydrofurane aus 3,4-Pentamethylenfuran und 2,2'-Bifu- tan. Dissertation, Universität Kiel, Germany 1995.
2. Sheldrick, G. M.: Program Package S H E L X T L - p l u s . Release 4.1.
Siemens Analytical X-Ray Instruments Inc., Madison (WI53719), USA 1990.
Zeitschrift für Kristallographie - New Crystal Structures 213, 291-292 291
© by R. Oldenbourg Veriag, München 1998
C r y s t a l s t r u c t u r e o f m e t h y l ( 2 / г * , З S * , 4 5 * , 5 / г * ) - 2 , 3 , 4 , 5 - t e t r a h y d r o - 3 , 4 - d i h y d r o x y - 5 - m e t h o x y - 6 - o x o - 2 , 4 - h e p t a n o f u r a n - 3 - c a r b o x y I a t e ,
С 1 1 Н 1 4 0 2 ( С 0 0 С Н з ) ( 0 С Н з ) ( 0 Н ) 2
к. Peters, Е.-М. Peters
Max-Planck-Institut fur Festkörperforschung, HeisenbergstraBe 1, D-70506 Stuttgan, Germany
F. Ott and W. Tochtermann
Christian-Albrechts-Universität Kiel, Institut für Organische Chemie, Olshausenstraße 40, D-24098 Kiel, Germany Received August 14, 1997, CSD-No. 409072
C 3 2 C 5 0
Table 1. Parameters used for the X-ray data collection
Crystal: colorless prism, size 0.75 χ 0.75 χ 0.5 mm Wavelength: Mo Ka radiation (0.71073 Â)
μ: 1.20 cm"'
Diffractometer: Siemens R3m/V
Scan mode: Wyckoff
measurement'· 293 К
2втах: 55°
ЩНк1)т>яие·· 3282 Criterion for Fo. F „ > 3 o ( F o )
iHparam)refine<r· 198
Program: SHELXTL-plus
Source of material: The title compound was prepared as described inref. 1.
C 1 4 H 2 2 O 7 ,
monoclinic,
P\2\ln\(No. 14),
a=14.906(3) Â b =7.064(2) Â, с =13.631(2) Â, β =95.88(1)°, V=1427.87Â , Z=4,
R(F) = 0 . 0 4 7 , Ry^iF) = 0 . 0 4 7 .Table 2. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X У ζ t/iso
H(2) 4e 0.8662(1) 0.0866(3) 0.5314(1) 0.08 H(3) 4e 0.811(2) 0.115(4) 0.708(2) 0.08(1) H(4) 4£ 0.648(2) -0.064(4) 0.680(2) 0.09(1) H(5) 4e 0.6390(1) -0.1527(3) 0.4496(1) 0.08 H(7A) 4e 0.8544(1) -0.1455(3) 0.3388(1) 0.08 H(7B) 4e 0.9594(1) -0.1205(3) 0.3486(1) 0.08 H(8A) 4e 0.9518(1) -0.4599(3) 0.4077(2) 0.08 H(8B) 4e 0.9580(1) -0.4121(3) 0.2%3(2) 0.08 H(9A) 4e 0.8446(2) -0.6409(4) 0.3309(2) 0.08 H(9B) 4e 0.8137(2) -0.4825(4) 0.2543(2) 0.08 H(IOA) 4e 0.7034(1) -0.5259(3) 0.3604(2) 0.08 H(IOB) 4e 0.7436(1) -0.3214(3) 0.3746(2) 0.08 H ( l l A ) 4e 0.7794(1) -0.6267(3) 0.5030(1) 0.08 H ( l l B ) Ae 0.8369(1) -0.4407(3) 0.5132(1) 0.08 H(12A) 4e 0.6536(1) -0.4424(3) 0.5364(1) 0.08 H(12B) 4e 0.7268(1) -0.4740(3) 0.6259(1) 0.08 H(32A) 4e 1.0260(1) -0.0578(4) 0.8487(2) 0.08 H(32B) Ae 1.0319(1) -0.2328(4) 0.7789(2) 0.08 H(32C) 4e 0.9645(1) -0.2348(4) 0.8599(2) 0.08 H(50A) 4e 0.5420(1) 0.2648(3) 0.4588(2) 0.08 H(50B) 4e 0.5365(1) 0.0692(3) 0.4041(2) 0.08 H(50C) 4e 0.6179(1) 0.2054(3) 0.3934(2) 0.08
Table 3. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X У ζ и и ί/22 t/33 Un ί/13 ί/23
0 ( l ) 4e 0.75561(8) -0.0199(2) 0.45236(9) 0.0324(7) 0.0471(9) 0.0356(7) 0.0025(6) 0.0027(5) 0.0073(6) C(2) 4e 0.8351(1) -0.0319(3) 0.5212(1) 0.0311(9) 0.033(1) 0.034(1) -0.0001(8) 0.0033(7) 0.0030(8) C(3) 4e 0.8020(1) -0.0904(3) 0.6209(1) 0.0309(9) 0.0294(9) 0.033(1) 0.0012(7) 0.0029(7) -0.0007(8) 0(3) 4e 0.77491(9) 0.0770(2) 0.6676(1) 0.0382(7) 0.0368(8) 0.0408(8) 0.0038(6) 0.0000(6) -0.0082(7) C(4) 4e 0.7113(1) -0.1%2(3) 0.5885(1) 0.0290(9) 0.036(1) 0.034(1) -0.0007(8) 0.0048(7) -0.0006(8) 0(4) 4e 0.65247(9) -0.1859(2) 0.6639(1) 0.0349(7) 0.0418(9) 0.0403(8) -0.0000(6) 0.0106(6) 0.0004(7) C(5) 4e 0.6774(1) -0.0788(3) 0.4956(1) 0.0310(9) 0.037(1) 0.036(1) 0.0015(8) 0.0018(7) 0.0005(8) 0(5) 4e 0.62554(9) 0.0744(2) 0.5209(1) 0.0388(7) 0.0463(9) 0.0409(8) 0.0113(6) 0.0010(6) 0.0004(7)
2 9 2 C l i H i 4 0 2 ( C 0 0 C H 3 ) ( C X : H 3 ) ( 0 H ) 2 Table 3. (Continued)
Atom Site X г Un С/22 U}ì Un C/13 C/23
C(6) 4e 0.9098(1) -0.1503(3) 0.4819(1) 0.0332(9) 0.032(1) 0.039(1) -0.0043(8) 0.0072(8) 0.0034(8) 0(6) 4e 0.97326(8) -0.1%5(2) 0.5407(1) 0.0329(7) 0.0464(9) 0.0453(8) 0.0023(6) 0.0037(6) 0.0034(7) C(7) 4e 0.9104(1) -0.1888(3) 0.3724(1) 0.041(1) 0.053(1) 0.036(1) -0.000(1) 0.0111(8) 0.0030(9) C(8) 4e 0.9215(1) -0.4010(3) 0.3501(2) 0.052(1) 0.060(2) 0.043(1) 0.008(1) 0.013(1) -0.008(1) C(9) 4e 0.8330(2) -0.5080(4) 0.3223(2) 0.066(1) 0.055(2) 0.045(1) -0.002(1) 0.014(1) -0.013(1) C(10) 4e 0.7560(1) -0.4540(3) 0.3837(2) 0.049(1) 0.048(1) 0.042(1) -0.006(1) 0.0060(9) -0.008(1) C ( l l ) 4e 0.7781(1) -0.4919(3) 0.4940(1) 0.047(1) 0.032(1) 0.044( 1 ) -0.0002(9) 0.0079(9) -0.0026(9) C d 2) 4e 0.7134(1) -0.4098(3) 0.5641(1) 0.037(1) 0.033(1) 0.041(1) -0.0045(8) 0.0057(8) -0.0005(9) C(30) 4e 0.8711(1) -0.1996(3) 0.6905(1) 0.0307(9) 0.040(1) 0.0307(9) 0.0014(8) 0.0063(7) 0.0021(8) 0(30) Ле 0.87714(9) -0.3681(2) 0.6989(1) 0.0433(8) 0.0369(8) 0.0476(9) 0.0025(6) 0.0031(6) 0.0081(7) 0(31) 4e 0.92366(9) -0.0750(2) 0.7443(1) 0.0379(7) 0.0437(9) 0.0420(8) 0.0026(6) -0.0062(6) -0.0027(7) C(32) 4e 0.9922(1) -0.1569(4) 0.8138(2) 0.048(1) 0.068(2) 0.050(1) 0.004(1) -0.015(1) -0.001(1) C(50) 4e 0.5764(1) 0.1604(3) 0.4375(2) 0.045(1) 0.047(1) 0.054(1) 0.007(1) -0.002(1) 0.010(1)
References
1. Ott, F.: Synthese von Uberbnickten Methylüiranosiden und Studien zur säurekatalysieiten Racemisierung eines optisch aktiven Epoxyoxepins.
Dissertation, Universität Kiel, Germany 199S.
2. Sheldrick, G. M.: Program Package SHELXTL-plus. Release 4.1.
Siemens Analytical X-Ray Instruments Inc., Madison (WI53719), USA 1990.