Zeitschrift für Kristallographie - New Crystal Structures 213,285-286 285
© by R. Oldenbourg Vertag, München 1998
Crystal structure of (3a/î*,45*,8S*,8a/?*)-dimethyl decahydro-8-hydroxy·
(2'-oxapropane-diyliden)azulene-3a,4-dìcarboxyIate, CieHioOô
к. Peters, Е.-М. Peters
Max-Planck-Institui für Festkörperforschung. Heisenbergstraße 1. D-70506 Stuttgart. Germany T. Panitzsch and W. Tochtermann
Christian-Albrechts-Universität Kiel, Institut für Organische Chemie, Olshausenstraße 40, D-24098 Kiel, Germany Received April 25. 1997, CSD-No. 409015
C1 C2
Source of material: The title compound was prepared as described in ref. 1.
The asymmetric unit of t y s crystal structure is a pair of enanti- omers. These molecules are connected by the hydrogen bonds 0 8 - H 8 b - 0 1 4 ; d ( 0 8 - 0 1 4 ) = 297.3 pm. d(014-H8b) = 237 pm and 0 8 - H 8 d · · 0 1 4 ; d ( 0 8 - 0 1 4 ) = 309.0 pm and d ( 0 1 4 - H8d) = 227 pm.
C16H20O6, monoclinic,/Ί2ι/γ1 (No. 14), α =8.672 =22.448(1) Â, с =15.586(1) Â, β =102.92°, V=2957.3 Â ^ Z=8, R(F) =0.063, R'MF) =0.063.
Table 1. Parameters used for the X-ray data collection
Crystal: colorless prism, size 0.4 χ 0.4 χ 0.2 mm Wavelength: Mo Ka radiation (0.71073 Â)
μ :
I.IOcm"'
Diffractometer: Siemens P4
Scan mode: ω
T^meosuremenl'· 293 К
2 9 m a x :
55°
nhklUiçue: 6793 Criterion for Fo: f o > 3 a ( f o ) N(param)refined'· 404
Program: SHELXTL-plus
Table 2. Final atomic coordinates and displacement parameters (in Â^)
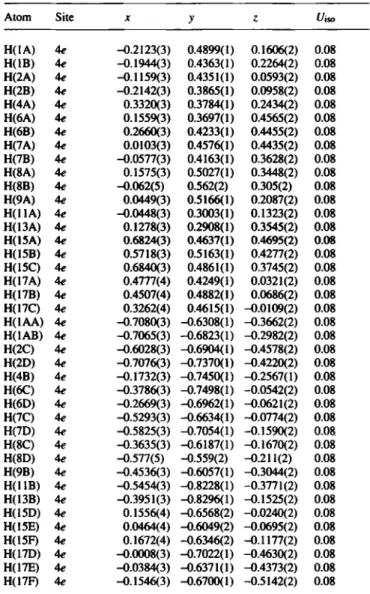
Atom Site X У ζ t/iso
H(IA) 4e -0.2123(3) 0.4899(1) 0.1606(2) 0.08
H(1B) 4e -0.1944<3) 0.4363(1) 0.2264(2) 0.08
H(2A) 4e -0.1159(3) 0.4351(1) 0.0593(2) 0.08
H(2B) 4e -0.2142(3) 0.3865(1) 0.0958(2) 0.08
H(4A) 4e 0.3320(3) 0.3784(1) 0.2434(2) 0.08
H(6A) 4e 0.1559(3) 0.3697(1) 0.4565(2) 0.08
H(6B) 4e 0.2660(3) 0.4233(1) 0.4455(2) 0.08
H(7A) 4e 0.0103(3) 0.4576(1) 0.4435(2) 0.08
H(7B) 4e -0.0577(3) 0.4163(1) 0.3628(2) 0.08
H(8A) 4e 0.1575(3) 0.5027(1) 0.3448(2) 0.08
H(8B) 4e -0.062(5) 0.562(2) 0.305(2) 0.08
H(9A) 4e 0.0449(3) 0.5166(1) 0.2087(2) 0.08
H(llA) 4e -0.0448(3) 0.3003(1) 0.1323(2) 0.08
H(13A) 4e 0.1278(3) 0.2908(1) 0.3545(2) 0.08
H(15A) 4e 0.6824(3) 0.4637(1) 0.4695(2) 0.08
H(15B) 4e 0.5718(3) 0.5163(1) 0.4277(2) 0.08
H(15C) 4e 0.6840(3) 0.4861(1) 0.3745(2) 0.08
H(17A) 4e 0.4777(4) 0.4249(1) 0.0321(2) 0.08
H(17B) 4e 0.4507(4) 0.4882(1) 0.0686(2) 0.08
H(17C) 4e 0.3262(4) 0.4615(1) -0.0109(2) 0.08
H(IAA) 4e -0.7080(3) -0.6308(1) -0.3662(2) 0.08
H(IAB) 4e -0.7065(3) -0.6823(1) -0.2982(2) 0.08
H(2C) 4e -0.6028(3) -0.6904(1) -0.4578(2) 0.08
H(2D) 4e -0.7076(3) -0.7370(1) -0.4220(2) 0.08
H(4B) 4e -0.1732(3) -0.7450(1) -0.2567(1) 0.08
H(6C) 4e -0.3786(3) -0.7498(1) -0.0542(2) 0.08
H(6D) 4e -0.2669(3) -0.6962(1) -0.0621(2) 0.08
H(7C) 4e -0.5293(3) -0.6634(1) -0.0774(2) 0.08
H(7D) 4e -0.5825(3) -0.7054(1) -0.1590(2) 0.08
H(8C) 4e -0.3635(3) -0.6187(1) -0.1670(2) 008
H(8D) 4e -0.577(5) -0.559(2) -0.211(2) 0.08
H(9B) 4e -0.4536(3) -0.6057(1) -0.3044(2) 0.08
H(llB) 4e -0.5454(3) -0.8228(1) -0.3771(2) 0.08
H(13B) 4e -0.3951(3) -0.8296(1) -0.1525(2) 0.08
H(15D) 4e 0.1556(4) -0.6568(2) -0.0240(2) 0.08
H(15E) 4e 0.0464(4) -0.6049(2) -0.0695(2) 0.08
H(15F) 4e 0.1672(4) -0.6346(2) -0.1177(2) 0.08
H(17D) 4e -0.0008(3) -0.7022(1) -0.4630(2) 0.08
H(17E) 4e -0.0384(3) -0.6371(1) -0.4373(2) 0.08
H(17F) 4e -0.1546(3) -0.6700(1) -0.5142(2) 0.08
286 С1бН2оОб
Table 3. Final atomic coordinates and displacement parameters (in Â^)
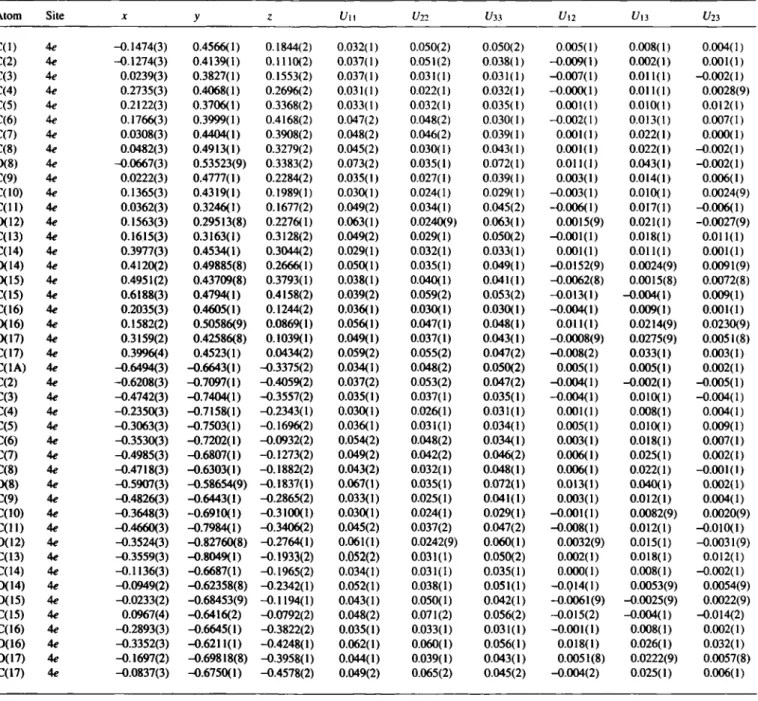
Atom Site X У ζ t/n U22 Í/33 Un Un Un
C d ) 4c -0.1474(3) 0.4566(1) 0.1844(2) 0.032(1) 0.050(2) 0.050(2) 0.005(1) 0.008(1) 0.004(1) C(2) 4e -0.1274(3) 0.4139(1) 0.1110(2) 0.037(1) 0.051(2) 0.038(1) -0.009(1) 0.002(1) 0.001(1) C(3) 4e 0.0239(3) 0.3827(1) 0.1553(2) 0.037(1) 0.031(1) 0.031(1) -0.007(1) 0011(1) -0.002(1) C(4) 4e 0.2735(3) 0.4068(1) 0.2696(2) 0.031(1) 0.022(1) 0.032(1) -0.000(1) 0011(1) 0.0028(9) C(5) 4e 0.2122(3) 0.3706(1) 0.3368(2) 0.033(1) 0.032(1) 0.035(1) 0.001(1) 0010(1) 0012(1) C(6) 4c 0.1766(3) 0.3999(1) 0.4168(2) 0.047(2) 0.048(2) 0.030(1) -0.002(1) 0013(1) 0.007(1) C(7) 4e 0.0308(3) 0.4404(1) 0.3908(2) 0.048(2) 0.046(2) 0.039(1) 0.001(1) 0.022(1) 0.000(1) C(8) 4c 0.0482(3) 0.4913(1) 0.3279(2) 0.045(2) 0.030(1) 0.043(1) 0.001(1) 0.022(1) -0.002(1) 0(8) 4c -0.0667(3) 0.53523(9) 0.3383(2) 0.073(2) 0.035(1) 0.072(1) 0.011(1) 0.043(1) -0.002(1) C{9) 4c 0.0222(3) 0.4777(1) 0.2284(2) 0.035(1) 0.027(1) 0.039(1) 0.003(1) 0014(1) 0.006(1) C(10) 4c 0.1365(3) 0.4319(1) 0.1989(1) 0.030(1) 0.024(1) 0.029(1) -0.003(1) 0010(1) 0.0024(9) C ( l l ) 4c 0.0362(3) 0.3246(1) 0.1677(2) 0.049(2) 0.034(1) 0.045(2) -0.006(1) 0.017(1) -0.006(1) 0(12) 4c 0.1563(3) 0.29513(8) 0.2276(1) 0.063(1) 0.0240(9) 0.063(1) 0.0015(9) 0.021(1) -0.0027(9) C(13) 4c 0.1615(3) 0.3163(1) 0.3128(2) 0.049(2) 0.029(1) 0.050(2) -0.001(1) 0018(1) 0011(1) C(14) 4c 0.3977(3) 0.4534(1) 0.3044(2) 0.029(1) 0.032(1) 0.033(1) 0.001(1) 0011(1) 0.001(1) 0(14) 4c 0.4120(2) 0.49885(8) 0.2666(1) 0.050(1) 0.035(1) 0.049(1) -00152(9) 0.0024(9) 0.0091(9) 0(15) 4c 0.4951(2) 0.43709(8) 0.3793(1) 0.038(1) 0.040(1) 0.041(1) -0.0062(8) 0.0015(8) 0.0072(8) C(15) 4c 0.6188(3) 0.4794(1) 0.4158(2) 0.039(2) 0.059(2) 0.053(2) -0013(1) -0.004(1) 0.009(1) C(16) 4c 0.2035(3) 0.4605(1) 0.1244(2) 0.036(1) 0.030(1) 0.030(1) -0.004(1) 0.009(1) 0.001(1) 0(16) 4c 0.1582(2) 0.50586(9) 0.0869(1) 0.056(1) 0.047(1) 0.048(1) 0011(1) 0.0214(9) 0.0230(9) 0(17) 4c 0.3159(2) 0.42586(8) 0.1039(1) 0.049(1) 0.037(1) 0.043(1) -0.0008(9) 0.0275(9) 0.0051(8) C(17) 4c 0.3996(4) 0.4523(1) 0.0434(2) 0.059(2) 0.055(2) 0.047(2) -0.008(2) 0.033(1) 0.003(1) C(1A) 4c -0.6494(3) -0.6643(1) -0.3375(2) 0.034(1) 0.048(2) 0.050(2) 0.005(1) 0.005(1) 0.002(1) C(2) 4c -0.6208(3) -0.7097(1) -0.4059(2) 0.037(2) 0.053(2) 0.047(2) -0.004(1) -0.002(1) -0.005(1) C(3) 4c -0.4742(3) -0.7404(1) -0.3557(2) 0.035(1) 0.037(1) 0.035(1) -0.004(1) 0.010(1) -0.004(1) C(4) 4c -0.2350(3) -0.7158(1) -0.2343(1) 0.030(1) 0.026(1) 0.031(1) 0.001(1) 0.008(1) 0.004(1) C(5) 4c -0.3063(3) -0.7503(1) -0.1696(2) 0.036(1) 0.031(1) 0.034(1) 0.005(1) 0010(1) 0.009(1) C(6) 4c -0.3530(3) -0.7202(1) -0.0932(2) 0.054(2) 0.048(2) 0.034(1) 0.003(1) 0.018(1) 0.007(1) C(7) 4c -0.4985(3) -0.6807(1) -0.1273(2) 0.049(2) 0.042(2) 0.046(2) 0.006(1) 0.025(1) 0.002(1) C(8) 4c -0.4718(3) -0.6303(1) -0.1882(2) 0.043(2) 0.032(1) 0.048(1) 0.006(1) 0.022(1) -0.001(1) 0(8) 4c -0.5907(3) -0.58654(9) -0.1837(1) 0.067(1) 0.035(1) 0.072(1) 0013(1) 0.040(1) 0.002(1) C(9) 4c -0.4826(3) -0.6443(1) -0.2865(2) 0.033(1) 0.025(1) 0.041(1) 0.003(1) 0012(1) 0.004(1) C(10) 4c -0.3648(3) -0.6910(1) -0.3100(1) 0.030(1) 0.024(1) 0.029(1) -0.001(1) 0.0082(9) 0.0020(9) C ( l l ) 4c -0.4660(3) -0.7984(1) -0.3406(2) 0.045(2) 0.037(2) 0.047(2) -0.008(1) 0012(1) -0010(1) 0(12) 4c -0.3524(3) -0.82760(8) -0.2764(1) 0.061(1) 0.0242(9) 0.060(1) 0.0032(9) 0015(1) -0.0031(9) C(13) 4c -0.3559(3) -0.8049(1) -0.1933(2) 0.052(2) 0.031(1) 0.050(2) 0.002(1) 0.018(1) 0012(1) C(14) 4c -0.1136(3) -0.6687(1) -0.1965(2) 0.034(1) 0.031(1) 0.035(1) 0.000(1) 0.008(1) -0.002(1) 0(14) 4c -0.0949(2) -0.62358(8) -0.2342(1) 0.052(1) 0.038(1) 0.051(1) -0.014(1) 0.0053(9) 0.0054(9) 0(15) 4c -0.0233(2) -0.68453(9) -0.1194(1) 0.043(1) 0.050(1) 0.042(1) -0.0061(9) -0.0025(9) 0.0022(9) C(15) 4c 0.0967(4) -0.6416(2) -0.0792(2) 0.048(2) 0.071(2) 0.056(2) -0.015(2) -0.004(1) -0.014(2) C(16) 4c -0.2893(3) -0.6645(1) -0.3822(2) 0.035(1) 0.033(1) 0.031(1) -0.001(1) 0.008(1) 0.002(1) 0(16) 4c -0.3352(3) -0.6211(1) -0.4248(1) 0.062(1) 0.060(1) 0.056(1) 0.018(1) 0.026(1) 0.032(1) 0(17) 4c -0.1697(2) -0.69818(8) -0.3958(1) 0.044(1) 0.039(1) 0.043(1) 0.0051(8) 0.0222(9) 0.0057(8) C(17) 4c -0.0837(3) -0.6750(1) -0.4578(2) 0.049(2) 0.065(2) 0.045(2) -0.004(2) 0.025(1) 0.006(1)
References
1. Panitzsch, T.: Synthese von Tremulanen und Merulanen. Dissertation, Universität Kiel, Germany 1997.
2. Sheldrick, G. M.: Program Package SHELXTL-plus. Release 4.1.
Siemens Analytical X-Ray Instruments Inc., Madison (WI53719), USA 1990.