ζ . Kristallogr. NCS 213 (1998) 4 9 7 - 4 9 8 4 9 7
© by R. Oldenbourg Verlag, München
C r y s t a l s t r u c t u r e o f ( l / г * , 2 5 * , 3 5 * , 4 / г * ) - 3 - b r o m o -
l , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 1 0 , l l , 1 2 , 1 3 Д 4 - t e t r a d e c a h y d r o - l , 4 - e p o x y -
b e n z o c y c l o d o d e c e n e - 2 - c a r b o x y l i c a c i d , С б Н 4 О ( С Н 2 ) 1 0 В г С О О Н
к . Peters, E.-M. Peters
Max-Planck-Institut für Festkörperforschung, Heisenbergstraße 1, D-70506 Stuttgart, Germany
A. Taugerbeck and W. Tochtermann
Christian-Albrechts-Universität, Insütut für Organische Chemie, OlshausenstraBe 40, D-24098 Kiel, Gennany Received October 1, 1997, CSD-No. 409111
C28 ^ ^ ^ C26
Source of material: The title compound was prepared as described in ref. 1.
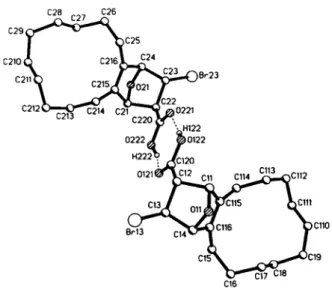
The two molecules are linked by hydrogen bridges through the carboxylic groups and together they form the asymmetric unit.
The corresponding Η atoms were located by means of a difference Fourier s y n t h e s i s and their p o s i t i o n s c o m p l e t e l y refíned [ í / ( H 1 2 2 - 0 1 2 2 ) = 76 pm; ¿ ( Н 2 2 2 - 0 2 2 2 ) = 9 2 pm; d ( H 1 2 2 - 0 2 2 1 ) = 190 pm; d ( H 2 2 2 - 0 1 2 1 ) = 177 pm]. The arrangement of these two molecules is approximately centrosymmetric.
С 1 7 Н 2 5 В Ю 3 , monoclinic, P2\ (No. 4), a =8.28(X2) Â,
b =18.317(4) Â, с =11.632(2) Â, β =107.08(3)°, V = 1 6 8 6 . 4 Â ^ Ζ =4, R(F) =0.067, R^F) =0.061.
Table 1. Parameters used for the X-ray data collection
Crystal: colorless plate, size 0.45 χ 0.65 χ 0.1 mm Wavelength: Mo Ka radiation (0.71073 Â)
μ: 24.50 cm"'
Diffractometer: Siemens P4
Scan mode: ω
'^measurement'· 293 К
2 0 m a x : 55°
ЩИк[)ипи,ие·· 7109
Criterion for Fo: F o > 3 a ( f o )
N(param)r^ed: 386
Program: SHELXTL-plus
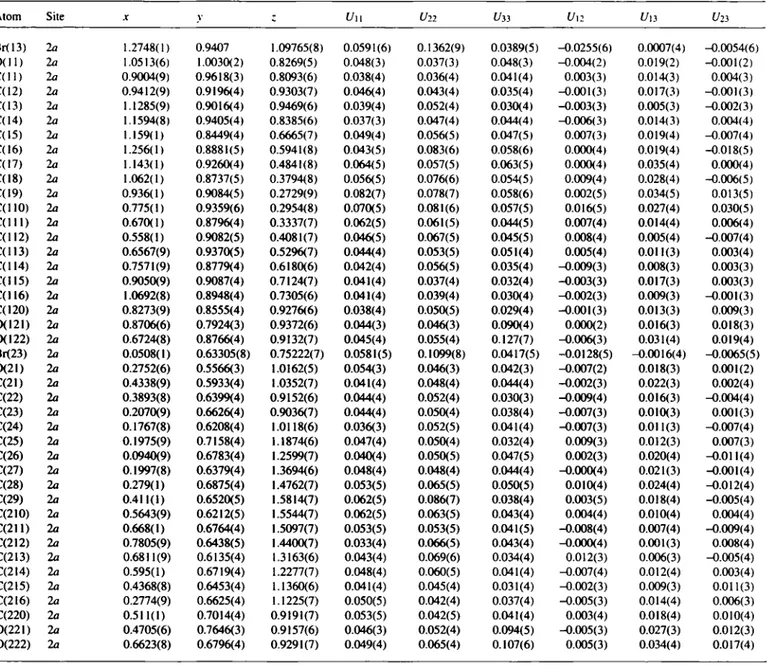
Table 2. Final atomic coordinates and displacement parameters (in A^)
Atom Site X У ζ í/i«>
H(ll) 2a 0.7954(9) 0.9880(3) 0.7893(6) 0.08 H(12) 2a 0.9226(9) 0.9459(4) 0.9%7(7) 0.08 H(13) 2a 1.1520(9) 0.8502(4) 0.9504(6) 0.08 H(14) 2a 1.2759(8) 0.9496(4) 0.8452(6) 0.08 H(15A) 2a 1.237(1) 0.8153(4) 0.7250(7) 0.08 H(15B) 2a 1.077(1) 0.8142(4) 0.6125(7) 0.08 H(16A) 2a 1.328(1) 0.8546(5) 0.5683(8) 0.08 H(16B) 2a 1.324(1) 0.9243(5) 0.6458(8) 0.08 H(17A) 2a 1.055(1) 0.9504(4) 0.5067(8) 0.08 H(17B) 2a 1.210(1) 0.9612(4) 0.4568(8) 0.08 H(18A) 2a 1.005(1) 0.8357(5) 0.4094(8) 0.08 H(18B) 2a 1.151(1) 0.8529(5) 0.3523(8) 0.08 H(19A) 2a 0.990(1) 0.9491(5) 0.2473(9) 0.08 H(19B) 2a 0.905(1) 0.8728(5) 0.2098(9) 0.08 H(llA) 2a 0.706(1) 0.9576(6) 0.2222(8) 0.08 H(llB) 2a 0.806(1) 0.9725(6) 0.3571(8) 0.08 H(llC) 2a 0.598(1) 0.8571(4) 0.2626(7) 0.08 H(llD) 2a 0.745(1) 0.8437(4) 0.3811(7) 0.08 H(llE) 2a 0.488(1) 0.8688(5) 0.4197(7) 0.08 H(llF) 2a 0.489(1) 0.9467(5) 0.3640(7) 0.08 Н(1Ю) 2a 0.7354(9) 0.9727(5) 0.5182(7) 0.08 H(llH) 2a 0.5787(9) 0.9597(5) 0.5653(7) 0.08 H(lll) 2a 0.7974(9) 0.8421(4) 0.5728(6) 0.08 H(HJ) 2a 0.6824(9) 0.8551(4) 0.6566(6) 0.08 H(122) 2a 0.61(1) 0.843(5) 0.906(9) 0.08(4) H(21) 2a 0.5354(9) 0.5649(4) 1.0515(7) 0.08 H(22) 2a 0.3985(8) 0.6142(4) 0.8455(6) 0.08 H(23) 2a 0.1898(9) 0.7145(4) 0.9044(7) 0.08 H(24) 2a 0.0599(8) 0.6139(4) 1.0068(6) 0.08 H(25A) 2a 0.2854(9) 0.7439(4) 1.2415(6) 0.08 H(25B) 2a 0.1245(9) 0.7477(4) 1.1294(6) 0.08 H(26A) 2a 0.0187(9) 0.6441(4) 1.2083(7) 0.08 H(26B) 2a 0.0294(9) 0.7149(4) 1.2859(7) 0.08 H(27A) 2a 0.1289(8) 0.6030(4) 1.3929(6) 0.08 H(27B) 2a 0.2887(8) 0.6128(4) 1.3483(6) 0.08 H(28A) 2a 0.331(1) 0.7277(4) 1.4480(7) 0.08 H(28B) 2a 0.190(1) 0.7054(4) 1.5060(7) 0.08 H(29A) 2a 0.358(1) 0.6130(5) 1.6114(7) 0.08 H(29B) 2a 0.450(1) 0.6884(5) 1.6429(7) 0.08 H(21A) 2a 0.6354(9) 0.5993(5) 1.6266(7) 0.08 H(21B) 2a 0.5270(9) 0.5844(5) 1.4936(7) 0.08 H(21C) 2a 0.592(1) 0.7105(4) 1.4583(7) 0.08 H(21D) 2a 0.739(1) 0.7018(4) 1.5783(7) 0.08 H(21E) 2a 0.8558(9) 0.6811(5) 1.4287(7) 0.08 H(21F) 2a 0.8448(9) 0.6047(5) 1.4866(7) 0.08 H(21G) 2a 0.5963(9) 0.5806(4) 1.3268(6) 0.08 H(21H) 2a 0.7581(9) 0.5874(4) 1.2836(6) 0.08 H(21D 2a 0.672(1) 0.6885(4) 1.1861(7) 0.08 Н(2Ш 2a 0.566(1) 0.7118(4) 1.2713(7) 0.08 H(222) 2a 0.73(2) 0.718(7) 0.92(1) 0.17(6)
498
СбНбО(СН2)10ВгС(Х)НTable 3. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X v Ζ Uu Un Uìì Un f/|3 Uli
Br(13) 2a 1.2748(1) 0.9407 1.09765(8) 0.0591(6) 0.1362(9) 0.0389(5) -0.0255(6) 0.0007(4) -0.0054(6) CHID 2a 1.0513(6) 1.0030(2) 0.8269(5) 0.048(3) 0.037(3) 0.048(3) -0.004(2) 0.019(2) -0.001(2) C ( l l ) 2a 0.9004(9) 0.9618(3) 0.8093(6) 0.038(4) 0.036(4) 0.041(4) 0.003(3) 0.014(3) 0.004(3) C(12) 2a 0.9412(9) 0.9196(4) 0.9303(7) 0.046(4) 0.043(4) 0.035(4) -0.001(3) 0.017(3) -0.001(3) C(13) 2a 1.1285(9) 0.9016(4) 0.9469(6) 0.039(4) 0.052(4) 0.030(4) -0.003(3) 0.005(3) -0.002(3) C(14) 2a 1.1594(8) 0.9405(4) 0.8385(6) 0.037(3) 0.047(4) 0.044(4) -0.006(3) 0.014(3) 0.004(4) C(15) 2a 1.159(1) 0.8449(4) 0.6665(7) 0.049(4) 0.056(5) 0.047(5) 0.007(3) 0.019(4) -0.007(4) C(16) 2a 1.256(1) 0.8881(5) 0.5941(8) 0.043(5) 0.083(6) 0.058(6) 0.000(4) 0.019(4) -0.018(5) C(I7) 2a 1.143(1) 0.9260(4) 0.4841(8) 0.064(5) 0.057(5) 0.063(5) 0.000(4) 0.035(4) 0.000(4) C(18) la 1.062(1) 0.8737(5) 0.3794(8) 0.056(5) 0.076(6) 0.054(5) 0.009(4) 0.028(4) -0.006(5) C(19) 2a 0.936(1) 0.9084(5) 0.2729(9) 0.082(7) 0.078(7) 0.058(6) 0.002(5) 0.034(5) 0.013(5) C(llO) 2a 0.775(1) 0.9359(6) 0.2954(8) 0.070(5) 0.081(6) 0.057(5) 0.016(5) 0.027(4) 0.030(5) C ( l l l ) 2a 0.670(1) 0.8796(4) 0.3337(7) 0.062(5) 0.061(5) 0.044(5) 0.007(4) 0.014(4) 0.006(4) C(112) 2a 0.558(1) 0.9082(5) 0.4081(7) 0.046(5) 0.067(5) 0.045(5) 0.008(4) 0.005(4) -0.007(4) C(1I3) 2a 0.6567(9) 0.9370(5) 0.5296(7) 0.044(4) 0.053(5) 0.051(4) 0.005(4) 0.011(3) 0.003(4) C(114) 2a 0.7571(9) 0.8779(4) 0.6180(6) 0.042(4) 0.056(5) 0.035(4) -0.009(3) 0.008(3) 0.003(3) C(I15) 2a 0.9050(9) 0.9087(4) 0.7124(7) 0.041(4) 0.037(4) 0.032(4) -0.003(3) 0.017(3) 0.003(3) C ( I I 6 ) 2a 1.0692(8) 0.8948(4) 0.7305(6) 0.041(4) 0.039(4) 0.030(4) -0.002(3) 0.009(3) -0.001(3) C(I20) 2a 0.8273(9) 0.8555(4) 0.9276(6) 0.038(4) 0.050(5) 0.029(4) -0.001(3) 0.013(3) 0.009(3) 0(121) 2a 0.8706(6) 0.7924(3) 0.9372(6) 0.044(3) 0.046(3) 0.090(4) 0.000(2) 0.016(3) 0.018(3) 0(122) 2a 0.6724(8) 0.8766(4) 0.9132(7) 0.045(4) 0.055(4) 0.127(7) -0.006(3) 0.031(4) 0.019(4) Br<23) 2a 0.0508(1) 0.63305(8) 0.75222(7) 0.0581(5) 0.1099(8) 0.0417(5) -0.0128(5) -0.0016(4) -0.0065(5) 0(21) 2a 0.2752(6) 0.5566(3) 1.0162(5) 0.054(3) 0.046(3) 0.042(3) -0.007(2) 0.018(3) 0.001(2) C(2I) 2a 0.4338(9) 0.5933(4) 1.0352(7) 0.041(4) 0.048(4) 0.044(4) -0.002(3) 0.022(3) 0.002(4) C(22) 2a 0.3893(8) 0.6399(4) 0.9152(6) 0.044(4) 0.052(4) 0.030(3) -0.009(4) 0.016(3) -0.004(4) C(23) 2a 0.2070(9) 0.6626(4) 0.9036(7) 0.044(4) 0.050(4) 0.038(4) -0.007(3) 0.010(3) 0.001(3) C(24) 2a 0.1767(8) 0.6208(4) 1.0118(6) 0.036(3) 0.052(5) 0.041(4) -0.007(3) O.Ol 1(3) -0.007(4) C(25) 2a 0.1975(9) 0.7158(4) 1.1874(6) 0.047(4) 0.050(4) 0.032(4) 0.009(3) 0.012(3) 0.007(3) C(26) 2a 0.0940(9) 0.6783(4) 1.2599(7) 0.040(4) 0.050(5) 0.047(5) 0.002(3) 0.020(4) -0.011(4) C(27) 2a 0.1997(8) 0.6379(4) 1.3694(6) 0.048(4) 0.048(4) 0.044(4) -0.000(4) 0.021(3) -0.001(4) C(28) 2a 0.279(1) 0.6875(4) 1.4762(7) 0.053(5) 0.065(5) 0.050(5) 0.010(4) 0.024(4) -0.012(4) C(29) 2a 0.411(1) 0.6520(5) 1.5814(7) 0.062(5) 0.086(7) 0.038(4) 0.003(5) 0.018(4) -0.005(4) C(2I0) 2a 0.5643(9) 0.6212(5) 1.5544(7) 0.062(5) 0.063(5) 0.043(4) 0.004(4) 0.010(4) 0.004(4) C ( 2 I I ) 2a 0.668(1) 0.6764(4) 1.5097(7) 0.053(5) 0.053(5) 0.041(5) -0.008(4) 0.007(4) -0.009(4) C(2I2) 2a 0.7805(9) 0.6438(5) 1.4400(7) 0.033(4) 0.066(5) 0.043(4) -0.000(4) 0.001(3) 0.008(4) C(213) 2a 0.6811(9) 0.6135(4) 1.3163(6) 0.043(4) 0.069(6) 0.034(4) 0.012(3) 0.006(3) -0.005(4) C(2I4) 2a 0.595(1) 0.6719(4) 1.2277(7) 0.048(4) 0.060(5) 0.041(4) -0.007(4) 0.012(4) 0.003(4) C(215) 2a 0.4368(8) 0.6453(4) 1.1360(6) 0.041(4) 0.045(4) 0.031(4) -0.002(3) 0.009(3) 0.011(3) C(2I6) 2a 0.2774(9) 0.6625(4) 1.1225(7) 0.050(5) 0.042(4) 0.037(4) -0.005(3) 0.014(4) 0.006(3) C(220) 2a 0.511(1) 0.7014(4) 0.9191(7) 0.053(5) 0.042(5) 0.041(4) 0.003(4) 0.018(4) 0.010(4) 0(221) 2a 0.4705(6) 0.7646(3) 0.9157(6) 0.046(3) 0.052(4) 0.094(5) -0.005(3) 0.027(3) 0.012(3) 0(222) 2a 0.6623(8) 0.6796(4) 0.9291(7) 0.049(4) 0.065(4) 0.107(6) 0.005(3) 0.034(4) 0.017(4)
References
1. Taugerbeck, Α.: Stereoselektive Synthese optisch aktiver ftinktionalisier- ter Tetrahydrofurane - Modellsubstanzen fuer Neoglycolipide. Disserta- tion, Universität Юе1, Germany 1998.
2. Sheldrick, G. M.: Program Package SHELXTL-plus. Release 4.1.
Siemens Analytical X-Ray Instruments Inc., Madison (WI53719), USA 1990.