ζ. Kristallogr. NCS 213 (1998) 707-708 707
© by R. Oldenbourg Verlag, München
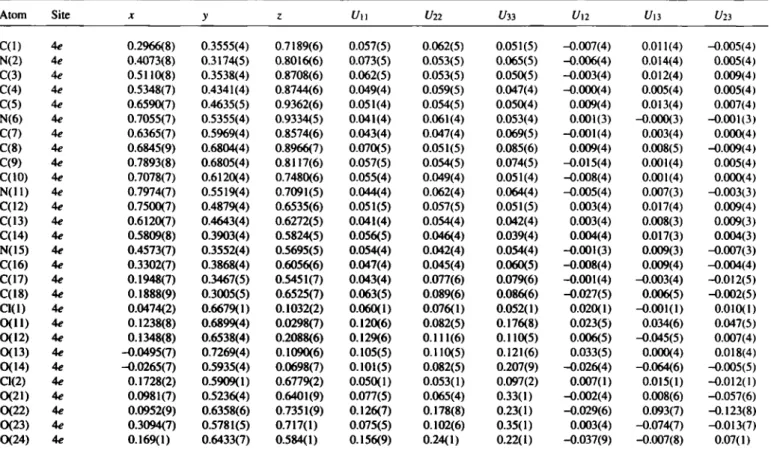
Crystal structure of (lS*,7/?*,105*,16Ä*)-2,6,ll,15-tetraaza-
tricyclo[14.2.0.0^'^®]octadeca-2,4,ll,13-tetraene bis(hydroperchlorate), (C7H, oN2)2(HC104)2
K. Peters, E.-M. Peters
Max-Planck-lnstitui für Festkörperforschung, Heisenbergstraße I, D-70506 Stuttgart, Germany
M. L. Werner and H. Quast
Universität Würzburg, Institut fìir Organische Chemie, Am Hubland, D-97074 Würzburg, Germany Received March 10, 1998, CSD-No. 409275
Source of material: The title compound was prepared, according to ref. 1, from c(s-l,2-cyclobutanediammonium dibromide and l,S-di- phenyl-I,5-diazapenta-l,34iiene hydroperchlorate in ethanol (dilute solution) in the presence of sodium acetate (3d, 298 K). Evaporation of the solvent yielded a yellow powder, which was recrystallized from methanol several times to afford colorless needles, mp 533 К (dec.), in 27% yield.
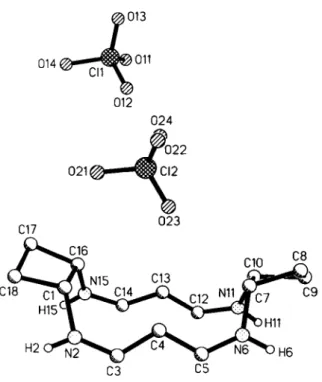
The cyclobutane rings of the title compound are connected via two parallel unsaturated chains showing open 6π systems with complete delocalization of the positive charge. The carbon-nitrogen bonds involving these chains adopt the Ζ confíguration while the carbon-carbon bonds exist in the £ configuration. Both cyclo- butane rings are slightly twisted. The lower homologue, which has cyclopropane instead of cyclobutane rings, shows a very similar structure (see ref. 2). One of the C104~ anions is highly disordered (C12 and 021-024). Refinements with split positions gave no im- provements.
C14H22CI2N4O8, monoclinic, P2\ln (No. 14), a =9.614(1) Â, b =17.013(3) Â, с =11.763(3) Â, β =100.00(1)°, V=1894.8 À^
Ζ =4, R(F) =0.086, Rvi(F) =0.087.
Table 1. Parameters used for the X-ray data collection
Crystal: colorless needle, si7f: O.IS χ 0.9 χ 0.1 nun Wavelength: Mo Ka radiation (0.71073 Â)
μ: 3.90 cm"'
Difñractometer: Siemens P4
Scan mode: ω
'^measunnunt'^ 293 К
2Θ™„: 55°
4352 Criterion for Fo. Fo>3o(F„)
^(рагат)гфтГ· 269
Program: SHELXTL-plus
Table 2. Final atomic coordinates and displacement parameters (in Â^)
Atom Site J: y ζ í/iso
H(l) 4€ 0.2733(8) 0.3926(4) 0.7741(6) 0.08 H(2) Ле 0.379(8) 0.273(5) 0.797(7) 0.08(3) H(3) 4e 0.5755(8) 0.3217(4) 0.9226(6) 0.08 H(4) 4e 0.4651(7) 0.4691(4) 0.8336(6) 0.08 H(5) 4e 0.7168(7) 0.4274(5) 0.9867(6) 0.08 H(6) 4e 0.783(7) 0.556(4) 0.974(6) 0.06(2) H(7) 4e 0.5402(7) 0.5800(4) 0.8510(6) 0.08 H(8A) 4e 0.6114(9) 0.7193(4) 0.8802(7) 0.08 H(8B) 4e 0.7281(9) 0.6839(4) 0.9763(7) 0.08 H(9A) 4e 0.8847(8) 0.6683(4) 0.8462(6) 0.08 H(9B) 4e 0.7881(8) 0.7278(4) 0.7669(6) 0.08 H(10) 4e 0.6469(7) 0.6169(4) 0.6745(6) 0.08 H(ll) 4e 0.890(7) 0.562(3) 0.722(5) 0.04(2) H(I2) 4e 0.8191(7) 0.4544(4) 0.6284(6) 0.08 H(13) 4e 0.5376(7) 0.4990(4) 0.6399(5) 0.08 H(I4) 4e 0.6569(8) 0.3615(4) 0.5588(5) 0.08 H(15) 4e 0.454(6) 0.310(3) 0.548(5) 0.03(2) H(I6) 4e 0.3492(7) 0.4418(4) 0.5975(6) 0.08 H(I7A) 4e 0.2071(7) 0.3152(5) 0.4799(7) 0.08 H(I7B) 4e 0.1166(7) 0.3818(5) 0.5233(7) 0.08 H(18A) 4e 0.0984(9) 0.3014(5) 0.6766(7) 0.08 H(I8B) 4e 0.2203(9) 0.2471(5) 0.6498(7) 0.08
7 0 8 (C7HI0N2)2(HC1O4)2
Table 3. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X У ζ í/ll Uli ί/зз í/12 Un {/23
C(l) 4e 0.2966(8) 0.3555(4) 0.7189(6) 0.057(5) 0.062(5) 0.051(5) -0.007(4) 0.011(4) -0.005(4) N(2) 4e 0.4073(8) 0.3174(5) 0.8016(6) 0.073(5) 0.053(5) 0.065(5) -0.006(4) 0.014(4) 0.005(4) C(3) 4e 0.5110(8) 0.3538(4) 0.8708(6) 0.062(5) 0.053(5) 0.050(5) -0.003(4) 0.012(4) 0.009(4) C(4) 4e 0.5348(7) 0.4341(4) 0.8744(6) 0.049(4) 0.059(5) 0.047(4) -0.000(4) 0.005(4) 0.005(4) C(5) 4e 0.6590(7) 0.4635(5) 0.9362(6) 0.051(4) 0.054(5) 0.050(4) 0.009(4) 0.013(4) 0.007(4) N(6) 4e 0.7055(7) 0.5355(4) 0.9334(5) 0.041(4) 0.061(4) 0.053(4) 0.001(3) -0.000(3) -0.001(3) C(7) 4e 0.6365(7) 0.5969(4) 0.8574(6) 0.043(4) 0.047(4) 0.069(5) -0.001(4) 0.003(4) 0.000(4) C(8) 4e 0.6845(9) 0.6804(4) 0.8966(7) 0.070(5) 0.051(5) 0.085(6) 0.009(4) 0.008(5) -0.009(4) C(9) 4e 0.7893(8) 0.6805(4) 0.8117(6) 0.057(5) 0.054(5) 0.074(5) -0.015(4) 0.001(4) 0.005(4) C(10) 4e 0.7078(7) 0.6120(4) 0.7480(6) 0.055(4) 0.049(4) 0.051(4) -0.008(4) 0.001(4) 0.000(4) N ( l l ) 4e 0.7974(7) 0.5519(4) 0.7091(5) 0.044(4) 0.062(4) 0.064(4) -0.005(4) 0.007(3) -0.003(3) C(12) 4e 0.7500(7) 0.4879(4) 0.6535(6) 0.051(5) 0.057(5) 0.051(5) 0.003(4) 0.017(4) 0.009(4) C(13) 4e 0.6120(7) 0.4643(4) 0.6272(5) 0.041(4) 0.054(4) 0.042(4) 0.003(4) 0.008(3) 0.009(3) C(14) 4e 0.5809(8) 0.3903(4) 0.5824(5) 0.056(5) 0.046(4) 0.039(4) 0.004(4) 0.017(3) 0.004(3) N(15) 4e 0.4573(7) 0.3552(4) 0.5695(5) 0.054(4) 0.042(4) 0.054(4) -0.001(3) 0.009(3) -0.007(3) C(16) 4e 0.3302(7) 0.3868(4) 0.6056(6) 0.047(4) 0.045(4) 0.060(5) -0.008(4) 0.009(4) -0.004(4) C(17) 4e 0.1948(7) 0.3467(5) 0.5451(7) 0.043(4) 0.077(6) 0.079(6) -0.001(4) -0.003(4) -0.012(5) C(18) 4e 0.1888(9) 0.3005(5) 0.6525(7) 0.063(5) 0.089(6) 0.086(6) -0.027(5) 0.006(5) -0.002(5) Cl(l) 4e 0.0474(2) 0.6679(1) 0.1032(2) 0.060(1) 0.076(1) 0.052(1) 0.020(1) -0.001(1) 0.010(1) 0 ( I I ) 4e 0.1238(8) 0.6899(4) 0.0298(7) 0.120(6) 0.082(5) 0.176(8) 0.023(5) 0.034(6) 0.047(5) 0(12) Ae 0.1348(8) 0.6538(4) 0.2088(6) 0.129(6) 0.111(6) 0.110(5) 0.006(5) -0.045(5) 0.007(4) 0(13) 4e -0.0495(7) 0.7269(4) 0.1090(6) 0.105(5) 0.110(5) 0.121(6) 0.033(5) 0.000(4) 0.018(4) 0(14) 4e -0.0265(7) 0.5935(4) 0.0698(7) 0.101(5) 0.082(5) 0.207(9) -0.026(4) -0.064(6) -0.005(5) a ( 2 ) 4e 0.1728(2) 0.5909(1) 0.6779(2) 0.050(1) 0.053(1) 0.097(2) 0.007(1) 0.015(1) -0.012(1) 0(21) Ae 0.0981(7) 0.5236(4) 0.6401(9) 0.077(5) 0.065(4) 0.33(1) -0.002(4) 0.008(6) -0.057(6) 0(22) 4e 0.0952(9) 0.6358(6) 0.7351(9) 0.126(7) 0.178(8) 0.23(1) -0.029(6) 0.093(7) -0.123(8) 0(23) 4e 0.3094(7) 0.5781(5) 0.717(1) 0.075(5) 0.102(6) 0.35(1) 0.003(4) -0.074(7) -0.013(7) 0(24) 4e 0.169(1) 0.6433(7) 0.584(1) 0.156(9) 0.24(1) 0.22(1) -0.037(9) -0.007(8) 0.07(1)
References
1. Werner, M. L.: Reaktionen von 1,2-Cyclobutandiaminen mit CaibonylVer- bindungen. Dissertation, Universität WUrzburg, Germany 198S.
2. Peters, K.; Peters, E.-M.; von Schnering, H. G.; Quast, H.; Stawitz, J.:
Crystal structure refinement of bishomo(dihydro)tetraaza[14]annulene bis{hydroperchlorate), Ci2Hi6N4{Ha04)2. Z. Kristallogr. 199 (1992) 284-286.
3. Sheldrick, G. M.: Program Package SHELXTL-plus. Release 4.1.
Siemens Analytical X-Ray Instruments Inc., Madison (WIS3719), USA 1990.