ζ . Kristallogr. NCS 213 (1998) 7 1 1 - 7 1 2
711
© by R. Oldenbourg Verlag, München
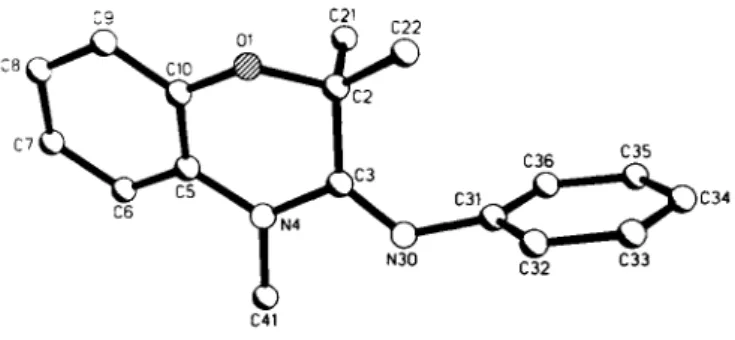
C r y s t a l s t r u c t u r e o f 3 , 4 - d i h y d r o - 2 , 2 , 4 - t r i m e t h y l - 3 - ( y V - p h e n y l i m i n o ) - 2 H - 1 , 4 - b e n z o x a z i n e , ( C H 3 ) 3 ( N C 6 H 5 ) C 8 H 4 N O
K. Peters, E.-M. Peters
Max-Planck-Institut fur Festkörperforschung. Heisenbergstraße 1. D-70506 Stuttgart, Germany
M. Ach and H. Quast
Universität Würzburg, Institut für Organische Chemie, Am Hubland, D-97074 Würzburg, Germany Received March 23, 1998, CSD-No. 409284
Table 1. Parameters used for the X-ray data collection
C 3 4
Source of material: The title compound was prepared by 1,3-di- polar cycloaddition of phenylazide and 2-isopropyHdene-3- methyl-2,3-dihydrobenzoxazole affording a spirocyclic [3+2]
cycloadduct (see ref. 1 and 2) and subsequent photochemical extrusion of molecular nitrogen from this cycloadduct (benzene solution, λ > 295 nm). Recrystallization from ethanol afforded colorless crystals, mp 354 К - 356 К (see ref. 3).
The title compound adopts the £ configuration. The 3-iminodihydro- benzoxazine structure shows that, on photoextrusion of molecular nitrogen from the spirocyclic precursor, the dihydro-oxazole ring undergoes ring-expansion by 1,2 shift of the oxygen atom. This photochemical course of the ring-expansion contrasts strikingly to the thermal extrusion of nitrogen, which affords a 2-(iV-phenyl- imino)dihydrobenzoxazine derivative by 1,2-shift of the tiitrogen atom, see ref 3. The thermal extrusion of molecular nitrogen with concomitant ring-expansion has already been reported for the corre- sponding unstable [3+2] cycloadduct of methanesulfonylazide (see ref. 4).
C17H18N2O, monoclinic, CU с (No. 15), a =18.369(7) A,
¿,=9.044(3) λ , с =17.69(1) Â, β =94.62(4)°, V=2929.5 Â ^ Ζ = 8 , R(F) =0.072, R^F) =0.056.
Table 3, Final atomic coordinates and displacement parameters (in A^)
Crystal: colorless plate, size 0.4 χ 0.55 χ 0.15 mm Wavelength: Mo Ka radiation (0.71073 A)
μ:
0.80 cm 'Diffractometer: Siemens R3m/V
Scan mode: Wyckoff
Tn^uren^: 293 К
55°
щтшщие·. 3391
Criterion for Fo: Fo>3o(Fo) fHparam)nfUKd·· 182
Program: SHELXTL-plus
Table 2. Final atomic coordinates and displacement parameters (in A^)
Atom Site X У ζ Í/Uo
H(6A) 8/ 0.1063(2) -0.2522(4) 0.2292(2) 0.08 H(7A) 8/ 0.0932(2) -0.3226(4) 0.1016(2) 0.08 H(8A) 8/ 0.0728(2) -0.1460(5) 0.0066(2) 0.08 H(9A) 8/ 0.0708(2) 0.1059(5) 0.0385(2) 0.08 H(21A) 8/ 0.2445(2) 0.2717(5) 0.2613(2) 0.08 H(2IB) 8/ 0.2222(2) 0.3030(5) 0.1755(2) 0.08 H(2IC) 8/ 0.2204(2) 0.1410(5) 0.2068(2) 0.08 H(22A) 8/ 0.1454(2) 0.4686(4) 0.2865(2) 0.08 H(22B) 8/ 0.0651(2) 0.4395(4) 0.2529(2) 0.08 H(22C) 8/ 0.1257(2) 0.4792(4) 0.1988(2) 0.08 H(32A) 8/ 0.0295(2) 0.4106(5) 0.4098(2) 0.08 H(33A) 8/ 0.0557(3) 0.6288(5) 0.4760(2) 0.08 H(34A) 8/ 0.1760(3) 0.6920(5) 0.5151(2) 0.08 H(35A) 8/ 0.2704(3) 0.5349(5) 0.4857(2) 0.08 H(36A) 8/ 0.2460(2) 0.3167(4) 0.4176(2) 0.08 H(41A) 8/ 0.0913(2) -0.1828(4) 0.3270(2) 0.08 H(4IB) 8/ 0.0624(2) -0.0587(4) 0.3788(2) 0.08 H(41C) 8/ 0.1466(2) -0.0867(4) 0.3782(2) 0.08
Atom Site X У г Un t/22 í/33 Un Un 1 / 2 3
0 ( 1 ) 8/ 0.0841(1) 0.2215(3) 0.1709(1) 0.074(2) 0.047(2) 0.050(2) -0.003(1) -0.011(1) 0.007(1)
C(2) 8/ 0.1331(2) 0.2651(4) 0.2365(2) 0.064(3) 0.047(2) 0.044(2) -0.010(2) 0.001(2) 0.002(2) C(3) 8/ 0.1176(2) 0.1683(4) 0.3037(2) 0.0+4(2) 0.044(2) 0.053(2) 0.003(2) 0.003(2) 0.004(2) N(4) 8/ 0.1043(2) 0.0204(3) 0.2861(2) 0.064(2) 0.040(2) 0.042(2) -0.003(2) 0.003(2) 0.005(2) C(5) 8/ 0.0977(2) -0.0300(4) 0.2101(2) 0.050(3) 0.043(2) 0.046(2) -0.002(2) 0.001(2) -0.001(2) C(6) 8/ 0.0994(2) -0.1782(4) 0.1904(2) 0.059(3) 0.046(3) 0.057(2) 0.005(2) -0.000(2) 0.001(2) C(7) 8/ 0.0910(2) -0.2198(4) 0.1148(2) 0.074(3) 0.052(3) 0.068(3) 0.003(2) 0.003(2) -0.015(2) C(8) 8/ 0ХЛ91(2) -0.1160(5) 0.0587(2) 0.072(3) 0.069(3) 0.048(2) -0.001(3) 0.002(2) -0.012(2)
712 (CH3)3(NC6H5)C8H4N0 Table 3. (Continued)
Atom Site X 3- ζ ί/ιι Un ί/зз UM t/|3 ί/23
C(9) 8/ 0.0782(2) 0.0324(5) 0.0775(2) 0.067(3) 0.066(3) 0.042(2) -0.006(2) 0.002(2) 0.005(2) C(10) 8/ 0.0874(2) 0.0741(4) 0.1527(2) 0.047(3) 0.046(3) 0.051(2) -0.003(2) 0.002(2) 0.000(2) C(21) 8/ 0.2124(2) 0.2431(5) 0.2183(2) 0.074(3) 0.096(3) 0.067(3) -0.026(3) 0.013(2) 0.001(2) C(22) 8/ 0.1157(2) 0.4280(4) 0.2443(2) 0.113(4) 0.048(3) 0.064(3) -O.Ol 1(3) -0.004(3) 0.011(2) N(30) 8/ 0.1186(2) 0.2029(3) 0.3733(2) 0.074(2) 0.050(2) 0.049(2) -0.008(2) 0.007(2) -0.002(2) C(31) 8/ 0.1351(2) 0.3413(4) 0.4067(2) 0.066(3) 0.046(2) 0.047(2) -0.006(2) 0.008(2) -0.000(2) C(32) 8/ 0.0794(2) 0.4357(5) 0.4249(2) 0.063(3) 0.065(3) 0.070(3) -0.004(3) 0.003(2) -0.009(2) C(33) 8/ 0.0948(3) 0.5644(5) 0.4644(2) 0.073(4) 0.066(3) 0.090(3) 0.004(3) 0.018(3) -0.011(3) C(34) 8/ 0.1654(3) 0.6023(5) 0.4873(2) 0.096(4) 0.059(3) 0.080(3) -0.005(3) 0.007(3) -0.017(2) C(35) 8/ 0.2208(3) 0.5095(5) 0.4698(2) 0.073(4) 0.076(3) 0.087(3) -0.015(3) -0.009(3) -0.013(3) C(36) 8/ 0.2066(2) 0.3798(4) 0.4296(2) 0.063(3) 0.062(3) 0.068(3) 0.003(2) 0.005(2) -0.006(2) C(41) 8/ 0.1009(2) -0.0860(4) 0.3478(2) 0.081(3) 0.048(2) 0.053(2) -0.001(2) 0.006(2) 0.007(2)
References
1. Ach, M.: 1.3-Dipolare Cycloadditìon cyclischer Keten-iVJIÍ-acetale mit Aziden. Dissertation, Univenität Wüizburg, Germany 1992.
2. Quast, H.; Ach, M.; Kindermann, M. К.; Rademacher, P.; Schindler, M.:
Synthese, NMR-Spektren und Photoelektronen-Spektren von cyclischen Keten-AfXacetalen (2-Alkyliden-JV-heterocyclen). Chem. Ber. 126 (1993)503-516.
3. Quast, H.; Ach, M.: Unpublished results.
4. Quast, H.; Ach, M.; Peters, E.-M.; Peters, K.; von Schnering, H. G.:
1,3-Dipolare Cycloaddition electrophiler Azide an cyclische Keten-NX acetale. - Stickstoff-Extrusion und Ringerweiterang der [3+2]-Cycload- dukte. Liebigs Ann. Chem. (1992) 1259-1269.
6. Sheldrick, G. M.: Program Package SHELXTL-plus. Release 4.1.
Siemens Analytical X-Ray Instruments Inc., Madison (WI53719). USA 1990.