ζ . Kristallogr. NCS 213 (1998) 7 0 3 - 7 0 4
703
© by R. Oldenbourg Verlag, München
C r y s t a l s t r u c t u r e o f l , 4 , 8 , l l - t e t r a a z a - 5 , 7 , 1 2 , 1 4 - t e t r a m e t h y l c y c l o t e t r a d e c a · 4 , 6 , 1 1 , 1 3 - t e t r a e n e b i s ( h y d r o p e r c h l o r a t e ) , ( C 7 H i 2 N 2 ) 2 ( H C 1 0 4 ) 2
K. Peters, E.-M. Peters
Max-Planck-Institut für Festkörperforschung, Heisenbergstraße 1, D-70506 Stuttgart, Germany
R. Reinhardt and H. Quast
Universität Würzburg, Institut für Organische Chemie, Am Hubland, D-97074 Würzburg, Germany Received March 2, 1998, CSD-No. 409245
Source of material: The title compound was prepared, according to ref. 1, from the corresponding fluorosulfonate, see ref. 2, and sodium Perchlorate in water. Recrystallization from water gave colorless crystals, mp 520-523 К (dec.), in quantitative yield.
The dication has Cs symmetry and shows two separate open 6 π systems with extensive delocalization of the positive charge. The carbon-nitrogen bonds involving the unsaturated chains adopt the Ζ configuration while the carbon-carbon bonds exist in the E configuration. Previously, a different, apparently incorrect con- figuration has been assigned to the dication (see ref. 3). The present results confirm the structure of the dication which has been proposed on the basis of NMR evidence (see ref. 2).
Table 3. Final atomic coordinates and displacement parameters (in Â^)
Ci4H2tìCl2N408, monoclinic, P2\lc (No. 14), a =8.768(2) Â, b =7.492(1) Â, с =15.680(3) Â, β =103.02(3)°, Κ=1003.5 Â ^ Ζ =2, R(F) =0.052, R^F) =0.055.
Table 1. Parameters used for the X-ray data collection
Crystal: colorless plate, size 0.2S χ 0.3 χ 0.1 mm Wavelength: Mo Ka radiation (0.71073 Â)
μ: 3.70 cm-'
Diffractometer: Siemens P4
Scan mode: ω
Tmeasurentf/a· 293 К 55°
1273 Criterion for Fo". Fo>3a(Fo)
148
Program: SHELXTL-plus
Table 2. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X У ζ í/iso
H(l) 4e 0.116(6) -0.626(7) 0.335(3) 0.08 H(2A) 4e 0.429(6) -0.537(6) 0.355(3) 0.08 H(2B) 4e 0.284(5) -0.469(6) 0.285(3) 0.08 H(3B) 4e 0.191(5) -0.275(6) 0.375(3) 0.08 H(3A) 4e 0.310(5) -0.353(7) 0.462(3) 0.08 H(4) 4e 0.379(6) -0.123(7) 0.344(3) 0.08 H(6) 4e 0.547(6) -0.341(7) 0.524(3) 0.08 H(51A) 4e 0.7441(5) -0.0058(6) 0.4335(3) 0.08 H(51B) 4e 0.5853(5) 0.0938(6) 0.3976(3) 0.08 H(51C) 4e 0.6462(5) -0.0462(6) 0.3388(3) 0.08 H(71A) 4e 0.9775(5) -0.1627(6) 0.6059(3) 0.08 H(71B) 4e 0.8582(5) -0.0170(6) 0.5604(3) 0.08 H(71C) 4e 0.9197(5) -0.1608(6) 0.5037(3) 0.08
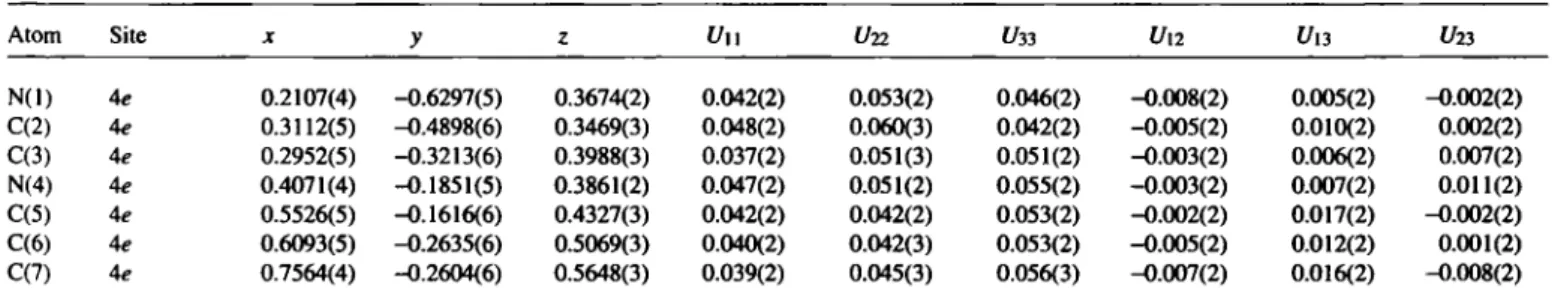
Atom Site X У ζ í/ll t/22 ί/зз Un í/13 í/23
N(l) 4e 0.2107(4) -0.6297(5) 0.3674(2) 0.042(2) 0.053(2) 0.046(2) -0.008(2) 0.005(2) -0.002(2) C(2) 4e 0.3112(5) -0.4898(6) 0.3469(3) 0.048(2) 0.060(3) 0.042(2) -0.005(2) 0.010(2) 0.002(2) C(3) 4e 0.2952(5) -0.3213(6) 0.3988(3) 0.037(2) 0.051(3) 0.051(2) -0.003(2) 0.006(2) 0.007(2) N(4) 4e 0.4071(4) -0.1851(5) 0.3861(2) 0.047(2) 0.051(2) 0.055(2) -0.003(2) 0.007(2) 0.011(2) C(5) 4e 0.5526(5) -0.1616(6) 0.4327(3) 0.042(2) 0.042(2) 0.053(2) -0.002(2) 0.017(2) -0.002(2) C(6) 4e 0.6093(5) -0.2635(6) 0.5069(3) 0.040(2) 0.042(3) 0.053(2) -0.005(2) 0.012(2) 0.001(2) C(7) 4e 0.7564(4) -0.2604(6) 0.5648(3) 0.039(2) 0.045(3) 0.056(3) -0.007(2) 0016(2) -0.008(2)
704
( C 7 H I 2 N 2 ) 2 ( H C 1 0 4 ) 2Table 3. (Continued)
Atom Site X У ζ í/ll í/22 ί/зз Ul2 Un t / 2 3
C(51) 4e 0.6402(5) -0.0170(6) 0.3976(3) 0.057(3) 0.054(3) 0.067(3) -0.005(2) 0.021(2) 0.009(2) C(71) 4e 0.8900(5) -0.1392(6) 0.5581(3) 0.046(2) 0.055(3) 0.081(3) -0.012(2) 0.014(2) 0.001(3) CI 4e 0.1975(1) 0.0222(2) 0.17667(7) 0.0472(6) 0.0770(9) 0.0565(6) -0.0026(6) 0.0085(5) 0.0107(7) 0 ( 1 ) 4e 0.1035(4) 0.1712(7) 0.1512(4) 0.064(2) 0.130(4) 0.204(5) 0.022(3) 0.017(3) 0.091(4) 0 ( 2 ) 4e 0.2153(8) -0.0692(7) 0.1037(3) 0.292(8) 0.147(5) 0.127(4) -0.093(5) 0.135(5) -0.064(4) СНЗ) 4e 0.3448(4) 0.0815(6) 0.2261(3) 0.055(2) 0.143(4) 0.105(3) -0.018(2) -0.001(2) 0.019(3) 0 ( 4 ) 4e 0.1249(4) -0.0884<6) 0.2302(2) 0.066(2) 0.140(4) 0.071(2) -0.022(2) 0.005(2) 0.047(2)
References
1. Reinhardt, R.: Reaktionen von 1,2-Cyclopropandianiinen mit Caibonyl- veibindungen. Zur Reaktion von Malondialdehyd mit Nucleinsaäure^- sen. Dissertation, Universität Würzburg, Germany I98S.
2. Lloyd, D.; McNab, H.; Marshall, D. R.: The Formation and Structure of l,S-Diaza- and S-Aza-l4>xapentadienium Salts and their Use in the Pre- paration of 2,3-Dihydro-l,4-<liazepinium Salts. J. Chem. Soc. Perkin Trans. I (1978) 1453-1460.
3. Riley, D. P.; Stone, J. Α.; Busch, D. H.: The Synthesis. Structure, and Properties of New Macrocyclic Ligands and Novel Sexadentate Iron Complexes Produced by Electrophilic Reactions of the Iron Derivatives.
J. Am. Chem. Soc. 98 (1976) 1752-1762.
4. Sheldrick, G. M.: Program Package SHELXTL-plus. Release 4.1.
Siemens Analytical X-Ray Instruments Inc., Madison (WI53719), USA 1990.