ζ . Kristallogr. NCS 213 (1998) 7 1 5 - 7 1 6
715
© by R. Oldenbourg Verlag, München
с "7
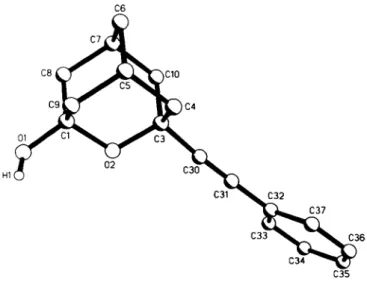
Crystal structure of 3-(phenylethinyl)-2-oxatricyclo[3.3.1.1 ' ]decan-l-ol, С9Н,20(0Н)(С2СбН5)
к . Peters, E. - M . Peters
Max-Planck-Institut für Festkörperforschung, Heisenbergstraße 1, D-70506 Stuttgart, Germany M. Witzel and H. Quast
Universität Würzburg, Institut für Organische Chemie, Am Hubland, D-97074 Würzburg, Germany Received April 22, 1998, CSD-No. 409297
C36 C35
Source of material: The title compound was prepared, according to ref. 1, from 3,7-bicyclo[3.3.1]nonanedione by the addition of pheny- lethinyllithium (tetrahydrofuran solutions, 195 К - 278 К, 2h).
Flash chromatography on silica gel with petroleum ether/ethyl ace- tate ( 1:1 ) of the crude product followed by recrystallization from the same solvent yielded colorless crystals, mp 420 К - 421 К, in 79%
yield.
The title compound is an example for the 2-oxadamantan-l-ols that always arise by the addition of nucleophiles at 3,7-bicy- clo[3.3.I]nonanedione. Rapid formation of the cyclic hemiacetal from the exo adduct of one nucleophile precludes addition of the second one (see ref. 2).
C17H18O2, monoclinic. Clic (No. 15), a =23.050(9) Л, b =6.291(2) Â, с =20.925(7) Â, β =112.39(3)°, V=2805.6 Â ^ Ζ =8, R(F) =0.044, Ry^F) =0.041.
Table 3. Final atomic coordinates and displacement parameters (in Л^)
Table 1. Parameters used for the X-ray data collection
Crystal: colorless plate, size 0.6 χ 0.8 χ 0.2 mm Wavelength: Mo Ka radiation (0.71073 A)
μ: 0.80 cm"'
Difftactometer: Siemens R3m/V
Scan mode: Wyckoff
'^measurement' 293 К
2втах: 55°
Щкк1)ип1чие·· 3242 Criterion for Fo. F„>3a(Fo)
^{param)refmei. 175
Program: SHF,I,XTL-plus
Table 2. Final atomic coordinates and displacement parameters (in Â^)
Atom Site JT у ζ í/iso
H(l) 8/ -0.048(1) -0.019(4) 0.937(1) 0.08 H(4A) 8/ 0.14926(9) 0.4698(3) 1.00045(9) 0.08 H(4B) 8/ 0.10258(9) 0.4028(3) 1.03515(9) 0.08 H(5A) 8/ 0.04864(9) 0.6103(3) 0.93613(9) 0.08 H(6A) 8/ 0.1100(1) 0.5219(3) 0.8717(1) 0.08 H(6B) 8/ 0.0389(1) 0.4844(3) 0.8262(1) 0.08 H(7A) 8/ 0.09773(9) 0.1921(3) 0.81739(9) 0.08 H(8A) 8/ 0.03949(8) -0.0797(3) 0.84500(8) 0.08 H(8B) 8/ -0.00478(8) 0.1109(3) 0.80912(8) 0.08 H(9A) 8/ -0.03496(8) 0.3716(3) 0.88343(9) 0.08 H(9B) 8/ -0.00916(8) 0.3380(3) 0.96368(9) 0.08 H(IOA) 8/ 0.15067(8) -0.0222(4) 0.91624(9) 0.08 H(IOB) 8/ 0.17971(8) 0.2067(4) 0.92772(9) 0.08 H(33A) 8/ 0.27701(9) -0.3486(3) 1.1384(1) 0.08 H(34A) 8/ 0.35987(9) -0.4303(4) 1.2431(1) 0.08 H(35A) 8/ 0.39714(9) -0.1775(4) 1.3299(1) 0.08 H(36A) 8/ 0.35231(9) 0.1613(4) 1.3141(1) 0.08 H(37A) 8/ 0.27003(8) 0.2478(3) 1.2101(1) 0.08
Atom Site X У ζ í/ll í/22 í/33 Un Un {/23
0(1) 8/ -0.03342(6) -0.0435(3) 0.90919(6) 0.0544(8) 0.0720(9) 0.0452(7) -0.0210(7) 0.0181(6) -0.0115(7) Cd) 8/ 0.01458(8) 0.0956(3) 0.91357(8) 0.0443(9) 0.050(1) 0.0328(8) -0.0043(8) 0.0123(7) -0.0013(7)
0(2) 8/ 0.06895(5) 0.0284(2) 0.97447(5) 0.0435(6) 0.0432(7) 0.0357(6) -0.0016(5) 0.0139(5) 0.0020(5)
C(3) 8/ 0.12533(8) 0.1530(3) 0.98553(8) 0.0415(9) 0.055(1) 0.0433(9) -0.0018(8) 0.0153(8) 0.0017(8) C(4) 8/ 0.11266(9) 0.3871(3) 0.99492(9) 0.059(1) 0.052(1) 0.053(1) -0.013(1) 0.0209(9) -0.0069(9) C(5) 8/ 0.05746(9) 0.4635(3) 0.93117(9) 0.074(1) 0.041(1) 0.059(1) 0.001(1) 0.027(1) 0.0046(9) C(6) 8/ 0.0737(1) 0.4377(3) 0.8663(1) 0.082(2) 0.066(1) 0.058(1) -0.005(1) 0.031(1) 0.017(1) C(7) 8/ 0.08759(9) 0.2037(3) 0.85770(9) 0.073(1) 0.071(1) 0.045(1) -0.002(1) 0.034(1) 0.004(1)
7 1 6 3-(Phenylethinyl)-2-oxatricyclo[3.3.1.1 ^'^Jdecan-1 -ol
Table 3. (Continued)
Atom Site X У ζ t/|l Í/22 Un Un и,} Uli
C(8) 8/ 0.03023(8) 0.0670(3) 0.84951(8) 0.067(1) 0.062(1) 0.0351(9) -0.002(1) 0.0206(8) -0.0037(9) C(9) 8/ 0.00039(8) 0.3251(3) 0.92302(9) 0.052(1) 0.055(1) 0.0446(9) 0.0107(9) 0.0153(8) 0.0033(8) C(IO) 8/ 0.14283(8) 0.1252(4) 0.92181(9) 0.056(1) 0.074(1) 0.061(1) 0.001(1) 0.034(1) 0.001(1) C(30) 8/ 0.17422(8) 0.0677(4) 1.04827(9) 0.044(1) 0.072(1) 0.053(1) 0.001(1) 0.0163(9) 0.003(1) C(31) 8/ 0.21520(8) 0.0154(4) 1.0997(1) 0.043(1) 0.075(1) 0.056(1) 0.002(1) 0.0178(9) 0.006(1) C(32) 8/ 0.26565(8) -0.0412(3) 1.16389(9) 0.0367(9) 0.066(1) 0.052(1) 0.0021(9) 0.0160(8) 0.007(1) C(33) 8/ 0.29234(9) -0.2432(3) 1.1740(1) 0.056(1) 0.064(1) 0.065(1) 0.003(1) 0.024(1) -0.006(1) C(34) 8/ 0.34134(9) -0.2912(4) 1.2362(1) 0.060(1) 0.057(1) 0.084(1) 0.016(1) 0.024(1) 0.012(1) C(35) 8/ 0.36326(9) -0.1426(4) 1.2873(1) 0.049(1) 0.073(2) 0.063(1) 0.006(1) 0.006(1) 0.012(1) C(36) 8/ 0.33691(9) 0.0574(4) 1.2779(1) 0.053(1) 0.069(1) 0.063(1) 0.001(1) 0.005(1) -0.004(1) C(37) 8/ 0.28829(8) 0.1083(3) 1.2165(1) 0.047(1) 0.056(1) 0.068(1) 0.007(1) 0.0135(9) 0.005(1)
References
1. Witzel, M.: Synthese und Cope-Umlagening disubstìtuierter Barbaralane.
Dissertation, Univeisität Würzburg, Germany 1994.
2. Quast, H.; Witzel, M.; Peters, E.-M.; Peters, К.; von Schnering, H. G.:
Synthesis and structures of 3,7-substituted bartiaralanes. Liebigs Ann.
Chem. (1995) 725-738.
3. Sheldrick, G. M.: Program Package SHELXTL-plus. Release 4.1.
Siemens Analytical X-Ray Instruments Inc., Madison (W153719), US A 1990.