Ζ. Kristallogr. N C S 2 1 5 ( 2 0 0 0 ) 2 2 3 - 2 2 4
© by Oldenbourg Wissenschaftsverlag, München
2 2 3
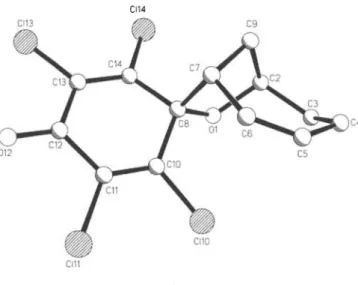
Crystal structure of 2,3,5,6-tetrachlorospiro[cyclohexa-2,5-diene-l,8- [7]oxabicyclo[4.2.1]non[2]ene]-4-one, [C5CI
40]C[C
7HIOO]
K. Peters*·
1, E.-M. Peters
1, M. Braun
11, Ο. Deeg
11and Μ. Christi
11 1 Max-Planck-Institut für Festkörperforschung, Heisenbergstraße 1, D-70506 Stuttgart, Germany" Universität Würzburg, Institut für Organische Chemie, Am Hubland, D-97074 Würzburg, Germany Received September 24, 1999, CCDC-No. 1267/293
CI14
Abstract
C13H10CI4O2, m o n o c l i n i c , P\2\!c\ ( N o . 14), a = 8 . 2 9 0 ( 1 ) Ä, b = 2 6 . 2 0 9 ( 4 ) Ä , c = 6 . 7 8 7 ( 1) Ä , β = 1 1 1 , 9 5 ( 1)°, V = 1 3 6 7 . 8 Ä3, Z = 4, Kgt(F) = 0 . 0 5 6 , wR(F) = 0 . 0 4 8 , T= 2 9 3 K.
Source of material
T h e title c o m p o u n d and t w o isomers thereof w e r e prepared by ir- radiation o f a s o l u t i o n o f chloranil and t r i c y c l o [ 4 . 1 . 0 . 0 '7]heptane or b i c y c l o [ 4 . 1 . 0 ] h e p t - 2 - e n e in b e n z e n e with v i s i b l e light [1, 2].
Table 1. Data collection and handling.
Crystal: pale yellow plate, size 0.4 χ 1.2 χ 0.07 mm Wavelength: Mo Ka radiation (0.71073 Ä)
μ: 8.60 cm"1
Diffractometer, scan mode: Bruker AXS P4, ω
20max: 55°
WiWjmeasured, N(hkl)Umquc: 4144,3115
Criterion for Fobs. N(hkl)gt: Fobs > 3 a(Fobs), 2040 N(param)Te fined: 172
Program: SHELXTL-plus [3]
Table 2. Atomic coordinates and displacement parameters (in A").
Atom Site X y ζ £/iso
H(2) 4e -0.5025(5) 0.4790(2) 0.7023(6) 0.08 H(3A) 4e -0.3314(6) 0.5139(2) 0.9751(7) 0.08 H(3B) 4e -0.1833(6) 0.4945(2) 0.9029(7) 0.08 H(4A) 4e -0.2763(6) 0.4539(2) 1.2237(7) 0.08 H(4B) 4e -0.0947(6) 0.4765(2) 1.2479(7) 0.08 H(5) 4e -0.0149(5) 0.3950(2) 1.2696(6) 0.08 H(6) 4e -0.1240(5) 0.3340(2) 1.0493(6) 0.08 H(7) 4e -0.3963(5) 0.3322(1) 0.8162(5) 0.08 H(9A) 4e -0.4791(5) 0.4109(2) 0.9747(6) 0.08 H(9B) 4e -0.5968(5) 0.3998(2) 0.7373(6) 0.08
Table 3. Atomic coordinates and displacement parameters (in Ä2).
Atom Site X y ζ Uii U22 t/33 ί/12 t/l3 t/23
Cl(10) 4e 0.0305(1) 0.40845(4) 0.7597(2) 0.0476(5) 0.0680(7) 0.0574(6) -0.0186(5) 0.0208(5) -0.0188(6) C l ( l l ) 4e 0.0954(2) 0.30223(5) 0.5876(2) 0.0619(7) 0.0826(9) 0.0788(8) 0.0316(6) 0.0288(6) -0.0015(7) Cl(13) 4e -0.5833(2) 0.28725(6) 0.1250(2) 0.0887(9) 0.095(1) 0.0516(6) -0.0484(8) 0.0063(6) -0.0139(7) Cl(14) 4e -0.6509(1) 0.38984(6) 0.3196(2) 0.0424(6) 0.115(1) 0.0664(7) 0.0193(7) 0.0101(5) 0.0197(7) O(l) 4e -0.3327(3) 0.43697(9) 0.6156(4) 0.065(2) 0.029(1) 0.058(2) 0.007(1) 0.037(1) 0.007(1) C(2) 4e -0.4099(5) 0.4549(2) 0.7645(6) 0.065(3) 0.040(2) 0.061(3) 0.019(2) 0.035(2) 0.009(2) C(3) 4e -0.2749(6) 0.4842(2) 0.9487(7) 0.093(3) 0.037(2) 0.077(3) 0.002(2) 0.047(3) -0.007(2) C(4) 4e -0.1909(6) 0.4561(2) 1.1599(7) 0.095(3) 0.047(3) 0.060(3) -0.005(3) 0.034(3) -0.010(2) C(5) 4e -0.1227(5) 0.4028(2) 1.1556(6) 0.058(3) 0.058(3) 0.043(2) -0.001(2) 0.022(2) 0.003(2) C(6) 4e -0.1880(5) 0.3654(2) 1.0200(6) 0.058(2) 0.038(2) 0.042(2) 0.005(2) 0.026(2) 0.006(2) C(7) 4e -0.3526(5) 0.3664(1) 0.8234(5) 0.048(2) 0.032(2) 0.045(2) -0.000(2) 0.024(2) 0.003(2) C(8) 4e -0.3174(4) 0.3835(1) 0.6201(5) 0.039(2) 0.029(2) 0.040(2) 0.001(2) 0.017(2) 0.003(2) C(9) 4e -0.4806(5) 0.4073(2) 0.8332(6) 0.051(2) 0.056(3) 0.059(3) 0.009(2) 0.030(2) 0.005(2) C(10) 4e -0.1384(4) 0.3677(1) 0.6292(5) 0.036(2) 0.037(2) 0.034(2) -0.005(2) 0.012(2) -0.001(2)
* Correspondence author
(e-mail: karpet@vsibm 1 .mpi-stuttgart.mpg.de)
224
[ C5C l 4 O ] C [ C7H i 0 O ]Table 3. Continued.
Atom Site X y ζ f / u f/22 t/33 t/12 C/13 C/23
C ( l l ) 4e -0.1102(4) 0.3237(1) 0.5502(5) 0.043(2) 0.039(2) 0.039(2) 0.008(2) 0.019(2) 0.003(2) C(I2) 4e -0.2525(6) 0.2927(2) 0.4068(6) 0.075(3) 0.033(2) 0.039(2) -0.003(2) 0.026(2) -0.000(2) 0(12) 4e -0.2270(4) 0.2519(1) 0.3378(5) 0.122(3) 0.038(2) 0.070(2) -0.003(2) 0.045(2) -0.011(2) C(13) 4e -0.4266(5) 0.3169(2) 0.3348(6) 0.060(3) 0.044(2) 0.033(2) -0.021(2) 0.010(2) -0.001(2) C(14) 4e -0.4528(4) 0.3595(2) 0.4228(6) 0.036(2) 0.053(3) 0.041(2) -0.002(2) 0.014(2) 0.015(2)
References
1. Braun, Μ.: Photochemische Cycloadditionen von Chloranil und anderen Carbonylverbindungen an Benzvalenen, Homobenzvalenen, Norbor- nadien, Norbonen und monocyclische Monoolefine. Dissertation, Universität Würzburg, Germany 1990.
2. Deeg, O.: Unpublished results. Universität Würzburg, Germany 1999.
3. Sheldrick, G. M.: Program Package SHELXTL-plus. Release 4.1.
Siemens Analytical X-Ray Instruments Inc., Madison (WI 53719), USA 1990.