460 Ζ. Kristallogr. NCS 213 (1998)
© by R. Oldenbourg Verlag, München
Crystal structure of cerium pentaphosphide, CePs
H. G. von Schnering, W. Wichelhaus, M. Wittmann, H. P. Weber and К. Peters
Max-Planck-Institut für Festkörperforschung, Heisenbergstraße 1, D-70569 Stuttgan. GermanyReceived December 17, 1997, transferred to 2nd update of database ICSD in 1998, CSD-No. 409181
CePs crystallizes in the space group
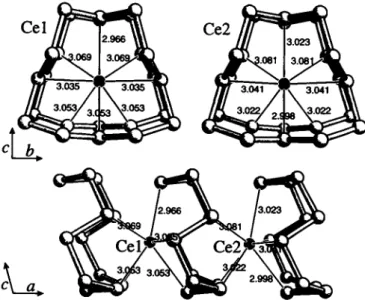
P2\lmisotypically to LaPs, a superstructure variant of NdPs (see ref. 1 ). The bond lengths vary between 2.134 Â and 2.226 Â for P - P and between 2.966 Â and 3.081 Â for Ce-P.
CePs, monoclinic, P12i/ml (No. 11), a =9.831(4) Â,
¿7=9.618(2) À, с =5.501(2) Â, β =104.21(3)°, V =504.2 À^
Ζ =4, R(F) =0.026, R^F) =0.037.
Table 1. Parameters used for the X-ray data collection
Source of material: The compound was formed by reaction of the elements in sealed glass or quartz ampoules in the presence of molten alkaline metal halides and small amounts of iodine (see ref. 1,2). The black prisms of CePj are stable against dilute bases and non-oxidizing acids. CeP5 is paramagnetic with 2.51 ць and semiconducting with Eg = 0.4 eV (see ref. 3).
Table 2. Final atomic coordinates and displacement parameters (in Â^)
Crystal: black, spherical, metallic luster, size 0.1 mm Wavelength: Mo Ka radiation (0.71073 Â)
μ: 104.3 cm"'
Diffractometer: SYNTEXPI
Scan mode: ω
'^measurement'· 293 К
2θπΐ3χ: 55°
Щ h k l) m i q ш • • 810
Criterion for Io. / ο > 2 σ ( / ο )
íi{param)refined·· 61
Program: SHELXTL-plus
Atom Site X У г ί/ιι U22 Un Un t/l3 Uli
Ce(l) 2e 0.0177(1) 1/4 0.3682(2) 0.0315(5) 0.0268(8) 0.0256(5) 0 0.0128(5) 0
Ce(2) 2e 0.5145(1) 1/4 0.3634(2) 0.0367(6) 0.0228(8) 0.0247(5) 0 0.0082(5) 0
P(l) 4 / 0.1887(3) 0.5912(3) 0.0435(4) 0.028(1) 0.027(2) 0.018(1) 0.005(1) 0.005(1) 0.001(1) P(2) 4 / 0.6918(3) 0.5887(3) 0.0347(5) 0.028(1) 0.020(2) 0.031(1) -0.002(1) 0.011(1) -0.002(1) P(3) 4 / 0.1447(3) 0.5389(4) 0.3934(5) 0.038(2) 0.028(3) 0.031(1) 0.003(2) 0.017(1) 0.001(2) P(4) 4 / 0.6472(3) 0.5372(3) 0.3965(5) 0.029(2) 0.027(3) 0.030(1) -0.002(2) 0.012(1) -0.001(2)
P(5) 2e 0.1458(4) 1/4 0.9157(6) 0.026(2) 0.040(4) 0.019(2) 0 0.011(1) 0
P(6) 2e 0.6429(4) 1/4 0.9223(7) 0.030(2) 0.013(2) 0.032(2) 0 0.008(2) 0
References
1. Wichelhaus, W.; von Schnering, H. G.: Die Pentaphosphide des Lanthans und Neodyms, LaPs und NdPs. Z. Anoi«. AUg. Chem. 419 (1976) 77-86.
2. Wittmann, M.: Darstellung, Struktur und Eigenschaften von Polyphos- phiden und Polyarseniden der Seltenerdmetalle. Dissertation, Universität Münster, Germany 1977.
3. Haitweg, M.: Über physikalische Eigenschaften der Seltenerd Pentaphos- phide LnPs, von CeP2 und LaAs2, sowie zur Kenntnis einiger neuer temärer Alkalimetall-Europium-Pnictide. Dissertation, Universität Stutt- gart, Germany 1987.
4. Sheldrick, G. M.; SHELXTL, an integrated system for solving, refining and displaying crystal structures from diffraction data. University of Göttingen, Germany 1978.