Ζ. Kristallogr. N C S 215 (2000) 4 3 - 4 4
© by O l d e n b o u r g W i s s e n s c h a f t s v e r l a g , M ü n c h e n
4 3
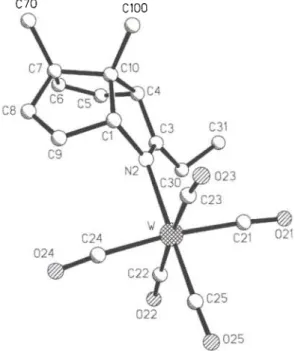
Crystal structure of 3-ethyI-7,10-dimethyl-2-azatricyclo[5.2.1.0 4,10 ]deca- 2,5,8-triene-iV-tungsten(0)-peiitacarbonyl, CisHnNOsW
K . P e t e r s * ·1, E . - M . P e t e r s1, T . D i e t z1 1 a n d H . Q u a s t "
' Max-Planck-Institut für Festkörperforschung, Heisenbergstraße 1, D-70506 Stuttgart, Germany
" Universität Würzburg, Institut für Organische Chemie, Am Hubland, D-97074 Würzburg, Germany Received July 29, 1999, CCDC-No. 1267/245
C 7 0 СЮО
Abstract
C 1 8 H 1 7 N O 5 W , m o n o c l i n i c , P\2\ln\ ( N o . 14), a = 1 0 . 7 5 7 ( 2 ) Ä, b = 2 6 . 3 5 1 (5) Ä , с = 6 . 5 2 5 8 ( 7 ) Ä , β = 9 0 . 8 5 ( 1)°, V = 1 8 4 9 . 6 Ä3, Ζ = 4 , Rgt(F) = 0 . 0 3 8 , wR(F) = 0 . 0 3 6 , Τ = 2 9 3 Κ .
Source of material
T h e title c o m p o u n d w a s p r e p a r e d , a c c o r d i n g t o [1], b y h e a t i n g of a s u s p e n s i o n o f e q u i m o l a r a m o u n t s o f 1 , 5 - d i m e t h y l s e m i - b u l l v a l e n e [2] a n d t r i s ( p r o p i o n i t r i l e ) t u n g s t e n t r i c a r b o n y l [3] in c y c l o h e x a n e f o r s e v e n d a y s u n d e r a r g o n a n d r e f l u x . T h e c o n v e r - s i o n w a s m o n i t o r e d b y p r o t o n N M R s p e c t r o s c o p y . F l a s h Table 3. Atomic coordinates and displacement parameters (in Ä2).
c h r o m a t o g r a p h y o f t h e d a r k b r o w n - b l a c k c r u d e p r o d u c t (silica gel, p e t r o l e u m e t h e r / e t h y l a c e t a t e , 9 5 : 5 ) a f f o r d e d a y e l l o w solid ( 2 0 % y i e l d , m p 4 8 7 K). R e c r y s t a l l i z a t i o n f r o m t h e s a m e s o l v e n t y i e l d e d t r a n s p a r e n t , y e l l o w c r y s t a l s .
Table 1. Data collection and handling.
Crystal:
Wavelength:
μ:
Diffractometer, scan mode:
20max:
N(hkl)measured, N(hkl)unique:
Criterion for fobs, N(hkl)p:
N(param) rermed:
Program:
yellow lump, size 0.1 χ 0.1 χ 0.85 mm Mo Ka radiation (0.71073 Ä) 62.70 cm"1
Siemens R3m/V, Wyckoff 55°
4707, 4250
Fobs > 3 σ (f o b s ) , 3 5 9 9 226
SHELXTL-plus [4]
Table 2. Atomic coordinates and displacement parameters (in Ä").
Atom Site X У г t/iso
H(l) 4e 0.4218(6) 0.0800(2) -0.117(1) 0.08 H(4) 4e 0.5534(6) 0.2071(2) -0.146(1) 0.08 H(5) 4e 0.6909(7) 0.2246(2) 0.153(1) 0.08 H(6) 4e 0.8264(6) 0.1534(3) 0.179(1) 0.08 H(8) 4e 0.7158(6) 0.0468(2) 0.211(1) 0.08 H(9) 4e 0.4965(6) 0.0352(2) 0.202(1) 0.08 H(30A) 4e 0.3171(7) 0.2187(2) 0.231(1) 0.08 H(30B) 4e 0.4521(7) 0.2327(2) 0.305(1) 0.08 H(31A) 4e 0.3602(9) 0.3001(3) 0.128(1) 0.08 H(31B) 4e 0.3403(9) 0.2655(3) -0.065(1) 0.08 H(31C) 4e 0.4752(9) 0.2795(3) 0.009(1) 0.08 H(70A) 4e 0.7746(7) 0.0596(3) -0.235(1) 0.08 H(70B) 4e 0.8760(7) 0.0771(3) -0.075(1) 0.08 H(70C) 4e 0.8309(7) 0.1144(3) -0.248(1) 0.08 H(10A) 4e 0.6461(8) 0.1407(3) -0.412(1) 0.08 H(10B) 4e 0.5009(8) 0.1367(3) -0.403(1) 0.08 H(10C) 4e 0.5825(8) 0.0874(3) -0.393(1) 0.08
Atom Site X У ζ U и Ul2 U33 С/12 С/13 t/23
W 4e 0.21525(2) 0.10973(1) 0.23986(4) 0.0468(1) 0.0623(1) 0.0484(1) 0.0075(1) 0.00430(9) 0.0023(1)
C(l) 4e 0.4757(6) 0.0968(2) -0.020(1) 0.050(3) 0.057(3) 0.056(3) -0.005(2) 0.008(3) -0.008(2)
N(2) 4e 0.3979(4) 0.1355(2) 0.0944(7) 0.046(2) 0.050(2) 0.047(2) 0.004(2) 0.005(2) 0.006(2)
C(3) 4e 0.4471(5) 0.1791(2) 0.0831(9) 0.054(3) 0.049(3) 0.057(3) 0.008(2) -0.002(3) 0.003(2)
C(4) 4e 0.5630(6) 0.1814(2) -0.043(1) 0.061(4) 0.051(3) 0.068(4) -0.004(3) 0.007(3) 0.007(3)
* Correspondence author
(e-mail: karpet@vsibml.mpi-stuttgart.mpg.de)
44
C 1 8 H 1 7 N O 5 WTable 3. Continued.
Atom Site X У ζ Un U22 ί/33 U η Un t/23
C(5) 4e 0.6771(7) 0.1926(2) 0.086(1) 0.066(4) 0.060(3) 0.095(5) -0.013(3) -0.001(4) -0.013(3)
C(6) 4e 0.7507(6) 0.1533(3) 0.099(1) 0.054(3) 0.079(4) 0.087(5) -0.006(3) -0.002(4) -0.009(4)
C(7) 4e 0.7067(6) 0.1080(2) -0.016(1) 0.047(3) 0.063(3) 0.065(4) 0.001(3) 0.002(3) -0.004(3)
C(8) 4e 0.6609(6) 0.0662(2) 0.124(1) 0.063(4) 0.065(3) 0.067(4) 0.016(3) 0.011(3) 0.002(3)
C(9) 4e 0.5391(6) 0.0595(2) 0.120(1) 0.064(4) 0.051(3) 0.076(4) 0.008(3) 0.018(3) 0.002(3)
C(10) 4e 0.5816(6) 0.1268(2) -0.1218(9) 0.049(3) 0.061(3) 0.051(3) -0.003(2) 0.003(3) -0.002(2)
C(21) 4e 0.1227(6) 0.1679(3) 0.111(1) 0.059(4) 0.085(4) 0.065(4) 0.007(3) 0.006(3) 0.006(3)
0(21) 4e 0.0650(6) 0.2017(2) 0.046(1) 0.096(4) 0.112(4) 0.123(5) 0.041(4) -0.004(4) 0.033(4)
C(22) 4e 0.2349(6) 0.1498(2) 0.497(1) 0.068(4) 0.072(3) 0.053(3) 0.013(3) 0.005(3) 0.004(3)
0(22) 4e 0.2416(6) 0.1716(2) 0.6519(8) 0.134(5) 0.098(3) 0.064(3) 0.020(3) -0.002(3) -0.014(3)
C(23) 4e 0.1760(6) 0.0664(2) -0.012(1) 0.049(3) 0.079(4) 0.062(4) 0.007(3) 0.003(3) -0.002(3)
0(23) 4e 0.1430(5) 0.0420(2) -0.1476(9) 0.081(4) 0.129(4) 0.085(4) 0.005(3) -0.010(3) -0.035(3)
C(24) 4e 0.2954(6) 0.0512(2) 0.3921(9) 0.056(3) 0.063(3) 0.056(3) -0.002(3) 0.005(3) 0.006(3)
0(24) 4e 0.3332(5) 0.0179(2) 0.4889(8) 0.093(4) 0.089(3) 0.094(4) 0.018(3) 0.018(3) 0.037(3)
C(25) 4e 0.0551(8) 0.0838(3) 0.349(1) 0.071(5) 0.096(5) 0.070(4) 0.007(4) 0.004(4) 0.020(4)
0(25) 4e -0.0379(5) 0.0707(3) 0.410(1) 0.056(3) 0.166(6) 0.111(5) -0.017(3) 0.021(3) 0.020(4)
C(30) 4e 0.4004(7) 0.2255(2) 0.187(1) 0.076(4) 0.052(3) 0.090(5) 0.005(3) 0.005(4) -0.005(3)
C(31) 4e 0.3925(9) 0.2718(3) 0.053(1) 0.132(8) 0.073(4) 0.120(7) 0.033(5) 0.022(7) 0.013(5)
C(70) 4e 0.8058(7) 0.0879(3) -0.157(1) 0.061(4) 0.084(4) 0.089(5) 0.007(4) 0.020(4) -0.002(4)
C(100) 4e 0.5779(8) 0.1225(3) -0.354(1) 0.073(5) 0.104(5) 0.052(4) -0.000(4) 0.006(4) 0.000(3)
References
1. Dietz, Т.: Synthese und Reaktionen von 1,5-Dialkylsemibullvalenen. Dis- sertation, Universität Würzburg, Germany 1995.
2. Quast, H.; Dietz,Т.; Peters, E.-M.; Peters, K.; von Schnering, H. G.: Opti- mised Preparation of 1,5-Dialkylsemibullvalenes. Liebigs Ann. Chem.
(1995) 1159-1168.
3. Kubas, G. J.: Preparation and Use of W(C0)3(NCR)3 (R= Et, Pr) as Im- proved Starting Materials for Syntheses of Tricarbonyl(ri -cyclo- heptatriene)tungsten and Other Substituted Carbonyl Complexes. Inorg.
Chem. 22 (1983) 692-694.
4. Sheldrick, G. M.: Program Package SHELXTL-plus. Release 4.1.
Siemens Analytical X-Ray Instruments Inc., Madison (WI 53719), USA 1990.