Ζ. Kristallogr. NCS 216 (2001) 105-107
© by O l d e n b o u r g W i s s e n s c h a f t s v e r l a g , M ü n c h e n
105
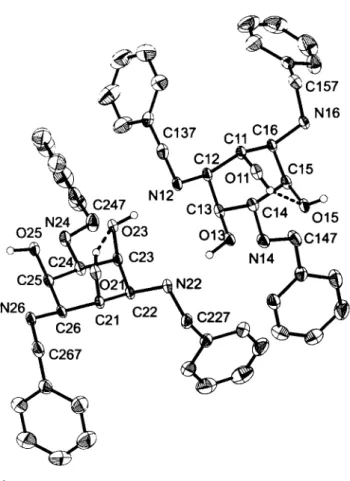
C r y s t a l s t r u c t u r e o f l , 3 , 5 - t r i s ( b e n z y l a m i n o ) - l , 3 , 5 - t r i d e o x y - c w - i n o s i t o l — m e t h a n o l ( 2 / 1 ) , ( C e H s C H z N H k C e H ^ O H ^ · 0 . 5 C H 3 ( ) H
J. Sander, V. Huch, M. Veith and K. Hegetschweiler
Universität des Saarlandes. Anorganische Chemie, Postfach 151150, D-66041 Saarbrücken, Germany Received July 14. 2000. CCDC-No. 1267/490
Abstract
C28H37N3O4, triclinic, P \ (No. 2), a = 13.100(3) Ä, b = 14.590(3) Ä , c = 15.190(3) Ä, α = 97.76(3)°, ß = 113.35(3)°, γ = 101.90(3)°, V = 2531.2 Ä3, Z = 2, RffiF) = 0.065, wRrefiF2) = 0.182, 7" = 2 9 3 K.
Source of material
l,3,5-tris(benzylamino)-l,3,5-trideoxy-m-inositol (tbci) w a s prepared by the reaction of 1,3,5-triamino-1,3,5-trideoxy-cii- inositol (taci) [1] with three equivalents of benzaldehyde in MeOH, followed by the reduction of the resulting Schiff base with NaBH4. Crystals of the title compound were grown from a solution of tbci in dry methanol.
Experimental details
Hydrogen atoms of the amino and hydroxy groups were located in a difference Fourier map and were refined with variable isotropic displacement parameters. The hydrogen atoms of the M e O H dis- ordered molecule were not considered. All other hydrogen atoms
were placed at calculated positions using a riding model with t/iso = 1.2i/eq of the corresponding heavy atom.
Discussion
1,3,5-triamino-1,3,5-trideoxy-ci.s-inositol (taci) and /V-alkylated derivatives have attracted considerable attention recently due to their interesting metal binding properties.[2] The three benzyl substituents of the title compound generate a rather lipophilic complexing agent and stabilize the chair conformation of the cyc- lohexane ring with three axial hydroxy groups. [3] The crystal structure analysis exhibited two crystallographically independent molecules in the asymmetric unit. Both of them have the expected chair conformation with axial hydroxy groups which is stabilized by intramolecular Ο — Η · · · 0 hydrogen bonding (O—O distance:
2.74 Ä and 2.73 Ä). T h e O—O distances between two axial oxy- gen atoms without hydrogen bonding are considerably longer and fall in the range of 2.88 Ä - 3.01 A (mean value: 2.94 Ä). The structure contains a disordered methanol molecule with two par- tially occupied positions (71(3) % and 29(3) % occupancy) for the hydroxy group. Both of the split positions are stabilized by an Ο—H—N hydrogen bond. Owing to this disorder,the observed C — Ο bond length is obviously not meaningful.
Table 1. Data collection and handling.
Crystal: colorless plate, size 0.1 χ 0.2 χ 0.3 mm Wavelength: Mo Ka radiation (0.71073 A)
μ: 0.81 cm"1
Diffractometer, scan mode: Stoe AED 2, ω/θ
29max: 47.96°
W!fc/)measured, N(hkl)uniquc· 7919,7919
Criterion for /0bS, N(hkl)gt: /obs > 2 σ ( ' ω , 5337
N(param)^ r„,ed: 672
Programs: SHELXS-97 [4], SHELXL-97 [5]
Table 2. Atomic coordinates and displacement parameters (in A2).
Atom Site X y ζ Uis„
H(13) 21 0.5900 0.2658 - 0 . 0 3 0 8 0.039 H ( l l ) 21 0.3060 0.0371 - 0 . 1 9 5 7 0.038
H(12) 21 0.4518 0.1668 -0.1752 0.037
H(14) 2i 0.4304 0.3191 -0.0786 0.040
H(15) 21 0.2742 0.2726 -0.0446 0.040
H(16) 2/ 0.2625 0.1825 -0.1871 0.037
* Correspondence author
(e-mail: hegetschweiler@mx.uni-saarland.de)
106
(C6H5CH2NH)3C6H9(OH)3 O.5CH3OHTable 2. Continued. Table 2. Continued.
Atom Site χ ν c f/ i s o Atom Site
H(37A) 2/ 0.4329 -0.0501 -0.1889 0.052
H(37B) 2 i 0.5578 -0.0380 -0.1816 0.052
H(47A) 2i 0.3765 0.3979 0.0893 0.060
H(47B) 2 i 0.4066 0.4517 0.0163 0.060
H(142) 2 i 0.7691 0.6628 0.2954 0.069
H( 141) 2i 0.6710 0.5206 0.1779 0.059
H(145) 2/ 0.3741 0.5531 0.1717 0.077
H(57A) 21 0.0017 0.0101 -0.2630 0.052
H(57B) 2/ 0.1124 0.0054 -0.2779 0.052
H(144) 2/ 0.4741 0.6947 0.2900 0.089
H(143) 21 0.6717 0.7519 0.3507 0.073
H( 131) 21 0.5970 0.1189 -0.2664 0.089
H(135) 2 j 0.3027 -0.0856 -0.3532 0.098
H(132) 2i 0.5319 0.1469 -0.4231 0.117
H(151) 2 i -0.0120 0.1986 -0.2558 0.076
H(97A) 2 i 0.2569 0.6831 0.1491 0.068
H(97B) 2 i 0.1625 0.5843 0.0882 0.068
H(27A) 2 i 0.9099 0.1780 0.2648 0.049
H(27B) 2 i 0.7805 0.1410 0.2469 0.049
H(221) 21 1.0107 0.3485 0.3297 0.068
H(225) 2/ 0.6735 0.2595 0.2610 0.066
H(155) 2/ 0.1102 0.0590 -0.4173 0.088
H(224) 2/ 0.6839 0.4104 0.3351 0.087
H(223) 2i 0.8549 0.5323 0.4040 0.099
H(133) 2 i 0.3536 0.0585 -0.5432 0.117
H(153) 2 i -0.0253 0.2713 -0.5021 0.115
H(152) 21 -0.0539 0.2973 -0.3627 0.108
H( 154) 2i 0.0625 0.1573 -0.5257 0.116
H(26) 2 i 0.1172 0.2607 0.1421 0.038
H(23) 2/ 0.7915 0.2805 -0.0012 0.039
H(21) 2 i -0.0021 -0.1459 -0.1775 0.038
H(22) 2 i 0.9363 0.2797 0.1499 0.036
H(25) 2 i 0.0983 0.2759 -0.0093 0.040
H(67A) 2i 0.2468 0.0693 0.1722 0.058
H(67B) 21 0.1368 0.0694 0.1898 0.058
H<24) 2 i -0.0204 0.3497 0.0282 0.042
H(245) 2 i 0.1683 0.4660 0.1743 0.084
H(222) 2; 1.0190 0.5013 0.4016 0.090
H(265) 21 0.1738 0.1597 0.3425 0.086
H(241) 2 i 0.2546 0.7361 0.3262 0.098
H(242) 2/ 0.2931 0.6804 0.4719 0.122
H(244) 2 i 0.2139 0.4121 0.3162 0.108
H(264) 2 i 0.7137 0.7204 0.5172 0.113
H(263) 21 0.5484 0.6159 0.5009 0.126
H(261) 2/ 0.3899 0.2515 0.2286 0.113
H(243) 2/ 0.2726 0.5193 0.4624 0.122
H(134) 2i 0.2396 -0.0581 -0.5089 0.128
H(262) 2/ 0.5005 0.6288 0.6312 0.153
H(22N) 2 1 0.769(3) 0.118(2) 0.103(2) 0.026(9) H(16N) 2i 0.150(3) 0.052(2) -0.120(2) 0.034(9) H(24N) 2 i 0.121(3) 0.745(2) 0.161(2) 0.031(9) H(26N) 2i 0.103(3) 0.088(3) 0.038(3) 0.06(1) H(130) 2/ 0.640(4) 0.202(3) 0.082(3) 0.07(1) H ( l l O ) 2i 0.368(3) 0.081(3) -0.006(3) 0.07(1) H(12N) 2 i 0.558(3) 0.063(3) -0.058(3) 0.06(1) H(210) 21 0.138(4) -0.072(3) 0.008(3) 0.07(1) H(14N) 21 0.523(3) 0.339(3) 0.115(3) 0.06(1) H(230) 21 0.704(4) 0.140(3) -0.082(3) 0.08(2) H(150) 21 0.282(4) 0.180(3) 0.049(3) 0.08(2) H(250) 2i 0.038(5) 0.151(4) -0.110(4) 0.12(2)
Table 3. Atomic coordinates and displacement parameters (in Ä2).
Atom Site Occ. χ y ζ U11 U22 Un υ π U2i
0(13) 2/ 0.5641(2) 0.1935(2) 0.0620(2) 0.025(1) 0.060(2) 0.039(1) 0.020(1) 0.015(1) 0.016(1) O ( l l ) 21 0.3520(2) 0.0303(2) -0.0574(2) 0.032(1) 0.039(1) 0.055(2) 0.015(1) 0.025(1) 0.021(1) C(13) 2 i 0.5228(2) 0.2255(2) -0.0269(2) 0.025(2) 0.038(2) 0.043(2) 0.011(1) 0.019(1) 0.016(2) N(14) 2i 0.5063(2) 0.3660(2) 0.0663(2) 0.030(2) 0.042(2) 0.050(2) 0.014(1) 0.017(1) 0.005(2) N(16) 2 i 0.1484(2) 0.0996(2) -0.1532(2) 0.021(1) 0.042(2) 0.041(2) 0.010(1) 0.016(1) 0.016(1) C ( l l ) 2 i 0.3433(2) 0.0835(2) -0.1313(2) 0.028(2) 0.039(2) 0.037(2) 0.014(1) 0.019(1) 0.012(1) C(12) 2/ 0.4632(2) 0.1405(2) -0.1171(2) 0.025(2) 0.039(2) 0.038(2) 0.014(1) 0.017(1) 0.012(1) CO 4) 21 0.4434(2) 0.2884(2) -0.0230(2) 0.029(2) 0.035(2) 0.040(2) 0.012(1) 0.017(1) 0.011(2) C(15) 2/ 0.3235(2) 0.2294(2) -0.0390(2) 0.026(2) 0.041(2) 0.043(2) 0.017(1) 0.019(1) 0.015(2) C(16) 21 0.2679(2) 0.1510(2) -0.1331(2) 0.022(2) 0.041(2) 0.036(2) 0.013(1) 0.016(1) 0.014(1) C(137) 21 0.4974(3) -0.0061(2) -0.1918(3) 0.038(2) 0.046(2) 0.055(2) 0.018(2) 0.028(2) 0.011(2) C(146) 2i 0.5106(3) 0.5201(2) 0.1600(2) 0.041(2) 0.038(2) 0.050(2) 0.014(2) 0.025(2) 0.010(2) C(147) 2 i 0.4399(3) 0.4320(2) 0.0775(3) 0.029(2) 0.049(2) 0.067(2) 0.013(2) 0.019(2) 0.003(2) C(142) 2 i 0.6888(3) 0.6407(3) 0.2701(3) 0.044(2) 0.054(2) 0.060(3) 0.008(2) 0.013(2) 0.012(2) C(141) 2 i 0.6298(3) 0.5554(3) 0.1996(3) 0.039(2) 0.055(2) 0.056(2) 0.017(2) 0.023(2) 0.009(2) C(136) 2 i 0.4576(3) 0.0131(3) -0.2927(3) 0.046(2) 0.055(2) 0.048(2) 0.022(2) 0.027(2) 0.006(2) C(145) 21 0.4543(3) 0.5747(3) 0.1961(3) 0.048(2) 0.056(2) 0.091(3) 0.011(2) 0.041(2) -0.004(2) C(157) 21 0.0748(3) 0.0489(2) -0.2572(2) 0.029(2) 0.047(2) 0.052(2) 0.011(2) 0.016(2) 0.011(2) C(144) 21 0.5141(4) 0.6598(3) 0.2669(3) 0.075(3) 0.065(3) 0.087(3) 0.015(2) 0.051(3) -0.011(2) C(143) 2i 0.6314(4) 0.6936(3) 0.3035(3) 0.077(3) 0.053(2) 0.047(2) 0.014(2) 0.025(2) 0.005(2) C(131) 2i 0.5242(4) 0.0824(3) -0.3150(3) 0.086(3) 0.074(3) 0.050(3) 0.001(3) 0.031(2) 0.004(2) C(135) 2 i 0.3504(4) -0.0385(4) -0.3664(3) 0.057(3) 0.121(4) 0.053(3) 0.008(3) 0.024(2) 0.008(3) 0(15) 2i 0.3367(2) 0.1914(2) 0.0459(2) 0.028(1) 0.065(2) 0.040(1) 0.016(1) 0.021(1) 0.018(1) 0(25) 2 i -0.0152(2) 0.1520(2) -0.0910(2) 0.031(1) 0.060(2) 0.041(1) 0.020(1) 0.021(1) 0.013(1) 0(23) 2; 0.7709(2) 0.1518(2) -0.0759(2) 0.023(1) 0.055(2) 0.041(1) 0.011(1) 0.017(1) 0.007(1) 0(21) 2/ 0.0922(2) -0.0593(2) -0.0460(2) 0.034(1) 0.041(1) 0.053(2) 0.015(1) 0.026(1) 0.015(1) N(26) 21 0.1475(2) 0.1419(2) 0.0888(2) 0.026(1) 0.051(2) 0.041(2) 0.020(1) 0.018(1) 0.017(1) N(12) 2i 0.5425(2) 0.0812(2) -0.1104(2) 0.027(1) 0.044(2) 0.042(2) 0.017(1) 0.020(1) 0.011(1) C(226) 2 i 0.8417(3) 0.2875(2) 0.2862(2) 0.043(2) 0.054(2) 0.033(2) 0.017(2) 0.022(2) 0.016(2)
(C6H5CH2NH)3C6H9(OH)3 · O.5CH3OH 107
Table 3. Continued.
Atom Site Occ. .V V UM U22 Uxy Ur. t/1.1 Ui3
C( 132) 2/ 0.4852(6) 0.0994(4) -0.4092(4) 0.143(5) 0.087(4) 0.067(3) 0.021(4) 0.056(4) 0.019(3) N(22) 2/ 0.7959(2) 0.1772(2) 0.1288(2) 0.027(1) 0.036(2) 0.042(2) 0.010(1) 0.023( 1) 0.010(1) C( 151) 2/ 0.0030(3) 0.1888(3) -0.3108(3) 0.058(2) 0.092(3) 0.071(3) 0.044(2) 0.038(2) 0.049(2) C(247i 2/ 0.1857(3) 0.6398(3) 0.1421(3) 0.057(2) 0.080(3) 0.065(3) 0.046(2) 0.040(2) 0.042(2) C(227) 2 i 0.8343(3) 0.1891(2) 0.2358(2) 0.040(2) 0.055(2) 0.042(2) 0.019(2) 0.027(2) 0.021(2) C(221) 2/ 0.9438(3) 0.3608(3) 0.3297(3) 0.052(2) 0.071(3) 0.044(2) 0.010(2) 0.024(2) 0.012(2) Cf 225) 2 i 0.7437(3) 0.3078(3) 0.2892(3) 0.050(2) 0.072(3) 0.046(2) 0.021(2) 0.025(2) 0.008(2) C(155) 2 i 0.0749(4) 0.1056(3) -0.4063(3) 0.077(3) 0.079(3) 0.054(3) 0.003(2) 0.033(2) 0.002(2) C(224) 2i 0.7501(4) 0.3981(4) 0.3333(3) 0.086(3) 0.090(3) 0.055(3) 0.052(3) 0.035(2) 0.009(2) C(223) 2 i 0.8520(5) 0.4709(4) 0.3750(3) 0.114(4) 0.069(3) 0.060(3) 0.032(3) 0.032(3) 0.004(2) C(133) 2 i 0.3798(6) 0.0472(5) -0.4802(4) 0.127(5) 0.123(5) 0.052(3) 0.059(4) 0.037(4) 0.026(3) C(153) 2 i -0.0039(5) 0.2338(5) -0.4567(4) 0.084(4) 0.113(5) 0.065(3) 0.003(3) 0.010(3) 0.050(3) C(152) 2 i -0.0231(4) 0.2478(4) -0.3752(4) 0.072(3) 0.116(4) 0.103(4) 0.046(3) 0.037(3) 0.069(4) C(154) 21 0.0464(5) 0.1651(5) -0.4712(4) 0.108(4) 0.114(5) 0.045(3) -0.013(4) 0.033(3) 0.016(3) N(24) 2/ 0.0954(2) 0.6893(2) 0.1178(2) 0.039(2) 0.059(2) 0.047(2) 0.026(2) 0.027(1) 0.028(2) C(26) 2/ 0.0707(2) 0.2021(2) 0.0891(2) 0.019(1) 0.042(2) 0.039(2) 0.012(1) 0.015(1) 0.011(1) C(23) 2 i 0.8399(2) 0.2373(2) -0.0002(2) 0.026(2) 0.042(2) 0.039(2) 0.017(1) 0.019(1) 0.013(2) C(21) 21 0.0307(2) -0.1532(2) -0.1095(2) 0.024(2) 0.042(2) 0.038(2) 0.014(1) 0.019(1) 0.015(2) C(22) 2 i 0.8893(2) 0.2176(2) 0.1024(2) 0.023(2) 0.038(2) 0.037(2) 0.009(1) 0.019(1) 0.012(1) C( 156) 2 i 0.0507(3) 0.1159(3) -0.3257(2) 0.029(2) 0.062(2) 0.038(2) 0.007(2) 0.012(2) 0.013(2) C(25) 2/ 0.0306(2) 0.2328(2) -0.0088(2) 0.023(2) 0.043(2) 0.040(2) 0.012(1) 0.017(1) 0.012(2) C(267) 2/ 0.1988(3) 0.1092(3) 0.1802(3) 0.036(2) 0.070(2) 0.056(2) 0.030(2) 0.026(2) 0.031(2) C(24) 2i -0.0603(3) 0.2880(2) -0.0212(2) 0.028(2) 0.041(2) 0.045(2) 0.013(1) 0.021(2) 0.017(2) C(266) 2 i 0.2705(3) 0.1898(3) 0.2701(3) 0.039(2) 0.088(3) 0.044(2) 0.033(2) 0.017(2) 0.027(2) C(246) 2i 0.2080(3) 0.6071(3) 0.2357(3) 0.039(2) 0.082(3) 0.058(2) 0.031(2) 0.028(2) 0.038(2) C(245) 2 i 0.1952(3) 0.5101(3) 0.2344(3) 0.054(2) 0.087(3) 0.079(3) 0.024(2) 0.028(2) 0.047(3) C(222) 2 i 0.9492(4) 0.4524(3) 0.3734(3) 0.081(3) 0.071(3) 0.054(3) 0.004(3) 0.022(2) 0.005(2) C(265) 2 i 0.2409(4) 0.2016(4) 0.3474(3) 0.071(3) 0.100(3) 0.053(3) 0.029(3) 0.030(2) 0.029(3) C(241) 2 i 0.2450(4) 0.6707(4) 0.3236(4) 0.081(3) 0.111(4) 0.071(3) 0.049(3) 0.036(3) 0.038(3) C(242) 2 i 0.2692(5) 0.6376(5) 0.4116(4) 0.090(4) 0.168(6) 0.067(3) 0.068(4) 0.033(3) 0.045(4) C(244) 2 i 0.2208(4) 0.4772(4) 0.3185(5) 0.076(3) 0.112(4) 0.101(4) 0.032(3) 0.041(3) 0.070(4) C(264) 2 i 0.6918(5) 0.7263(5) 0.5685(4) 0.100(4) 0.139(5) 0.051(3) 0.048(4) 0.031(3) 0.028(3) 0(51A) 2 i 0.71(3) 0.105(1) 0.4610(9) -0.0613(6) 0.129(7) 0.133(7) 0.095(5) -0.045(5) 0.077(5) -0.003(4) 0(51B) 2i 0.29 0.132(2) 0.430(3) -0.088(4) 0.10(1) 0.19(3) 0.23(4) 0.09(2) 0.01(2) 0.09(2) C(263) 2i 0.5948(5) 0.6638(5) 0.5587(4) 0.079(4) 0.142(5) 0.054(3) 0.024(4) 0.002(3) -0.004(3) C(261) 2 i 0.3681(4) 0.2557(4) 0.2800(3) 0.048(3) 0.152(5) 0.057(3) -0.002(3) 0.022(2) -0.003(3) C(243) 2i 0.2563(5) 0.5415(6) 0.4051(5) 0.083(4) 0.165(6) 0.100(5) 0.061(4) 0.051(4) 0.093(5) C(134) 2i 0.3122(5) -0.0218(5) -0.4598(4) 0.075(4) 0.166(6) 0.062(3) 0.025(4) 0.020(3) 0.019(4) C(262) 2 i 0.5662(5) 0.6726(5) 0.6359(4) 0.064(3) 0.192(7) 0.077(4) -0.018(4) 0.024(3) -0.018(4) C(51) 2 i 0.1409(6) 0.4936(5) -0.1273(6) 0.134(6) 0.111(5) 0.148(6) 0.040(4) 0.108(5) 0.056(4)
Acknowledgment. Financial support of the Deutsche Forschungs- gemeinschaft (Project No. HE 2799/2-2) is gratefully acknowledged.
References
1. Ghisletta, M.; Jalett, H. P.; Gerfin, T.; Grämlich, V.; Hegetschweiler, K.:
1,3,5-Triamino-1,3,5-trideoxy-c/s-inositol, a New Ligand with a Remark- able Versatility for Metal Ions. Part 2: Safe and Efficient Ligand Prepara- tion and Structure of the Free Ligand and the Co1" Complex. Helv. Chim.
Acta 75 (1992) 2233-2242.
2. Hegetschweiler, K.: A rigid, cyclohexane-based polyamino-polyalcohol as a versatile building block for tailored chelating agents. Chem. Soc. Rev.
28 (1999) 239-249.
3. Egli Α.: Synthese und Charakterisierung von Rhenium(V)-Inosit- und R h e n i u m ( I ) - C a r b o n y l - V e r b i n d u n g e n f ü r den E i n s a t z in d e r Nuklearmedizin. Dissertation No. 10936, ΕΤΗ Zürich, Switzerland 1994.
4. Sheldrick, G. M.: Phase annealing in SHELX-90: Direct Methods for Large Structure Crystallogr. A46 (1990) 467-473.
5. Sheldrick, G. M.: SHELXL-97, Program for Crystal Structure Refine- ment. University of Göttingen, Germany 1997.