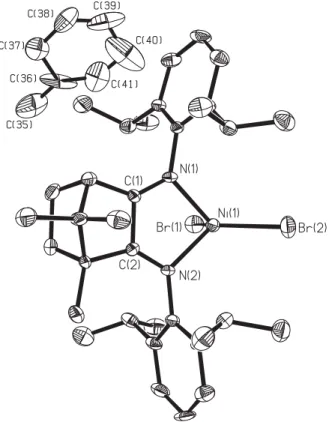
Crystal structure of [1R,7,7-trimethylbicyclo[2.2.1]heptan-2,3-bis(2,6- diisopropylphen-1-yl)imine]nickeldibromide]—toluene (1:1),
C 34 H 48 Br 2 N 2 Ni · C 7 H 8
Ch. Amort, B. Bildstein and K. Wurst*
University of Innsbruck, Institut of General, Inorganic and Theoretical Chemistry, Innrain 52a, A-6020 Innsbruck, Austria Received February 18, 2003, accepted and available on-line May 23, 2003; CCDC-No. 1267/1026
Abstract
C
41H
56Br
2N
2Ni, orthorhombic, P2
12
12
1(No. 19), a = 10.121(2) Å, b = 17.115(2) Å, c = 22.835(2) Å, V = 3955.5 Å
3, Z = 4, R
gt(F) = 0.037, wR
ref(F
2) = 0.086, T = 218 K.
Source of material
The Ni-complex was obtained from 1R-camphorchinon-N,N ¢ - bis(2,6-diisopropylphenyl)diimine [1] and (dimethoxyethane)- nickeldibromide.
Experimental details
The chiral compound crystallizes in the chiral space group P2
12
12
1and the absolute structure was determined by the method of Flack (Flack parameter x = 0.025(15)).
Discussion
In the asymmetric unit is one molecule of the complex and one molecule of the solvent toluene. The Ni(II) cation has a distorted tet- rahedral coordination with bond distances of 2.027(5) Å and 2.033(5) Å for Ni—N bonds and 2.294(2) Å and 2.328(2) Å for
Ni—Br bonds. The smallest and greatest bond angle is Ð N1–Ni2–N2 with 82.8(2) ° and Ð Br1–Ni1–Br2 with 123.5(1) ° . The double bonds of the imino groups are located between N1–C1 and N2–C2 with bond distances of 1.272(8) Å and 1.274(8) Å. The solvent toluene is slightly disordered and was refined without hydrogen atoms.
Z. Kristallogr. NCS
218(2003) 187–188 187
© by Oldenbourg Wissenschaftsverlag, München
Crystal: brown prism, size 0.35 × 0.5 × 0.65 mm Wavelength: MoKaradiation (0.71073 Å)
m: 25.40 cm–1
Diffractometer, scan mode: Bruker P4,w
2qmax: 40.98°
N(hkl)measured,N(hkl)unique: 3773, 3310 Criterion forIobs,N(hkl)gt: Iobs> 2s(Iobs), 2759 N(param)refined: 416
Programs: SHELXS-97 [2], SHELXL-97 [3], SHELXTL [4]
Table 1.Data collection and handling.
H(4A) 4a 1.0399 1.1343 0.4150 0.066
H(4B) 4a 0.9804 1.0564 0.3872 0.066
H(5A) 4a 0.9144 1.1256 0.3073 0.075
H(5B) 4a 0.9770 1.2031 0.3345 0.075
H(6) 4a 1.1167 1.1793 0.2503 0.054
H(8A) 4a 1.2658 1.0950 0.4457 0.078
H(8B) 4a 1.3495 1.0358 0.4073 0.078
H(8C) 4a 1.2191 1.0071 0.4391 0.078
H(9A) 4a 1.3571 1.0762 0.2739 0.095
H(9B) 4a 1.4323 1.1181 0.3260 0.095
H(9C) 4a 1.3884 1.1667 0.2703 0.095
H(10A) 4a 1.1622 1.2301 0.3798 0.113
H(10B) 4a 1.2687 1.2605 0.3344 0.113
H(10C) 4a 1.3140 1.2121 0.3900 0.113
H(13) 4a 1.1670 1.0934 0.0357 0.063
H(14) 4a 0.9492 1.1216 0.0159 0.072
H(15) 4a 0.7940 1.1110 0.0884 0.065
H(17) 4a 1.2993 1.0234 0.1690 0.051
H(18A) 4a 1.4493 0.9949 0.0943 0.101
H(18B) 4a 1.3483 1.0263 0.0468 0.101
H(18C) 4a 1.3148 0.9500 0.0832 0.101
H(19A) 4a 1.4512 1.1231 0.1435 0.093
H(19B) 4a 1.3192 1.1590 0.1693 0.093
H(19C) 4a 1.3449 1.1600 0.1008 0.093
H(20) 4a 0.8431 1.0432 0.2341 0.060
H(21A) 4a 0.6232 1.0123 0.2061 0.124
H(21B) 4a 0.7248 0.9616 0.1695 0.124
H(21C) 4a 0.6560 1.0351 0.1404 0.124
Table 2.Atomic coordinates and displacement parameters (in Å2)
.
Atom Site x y z Uiso
_____________
* Correspondence author (e-mail: klaus.wurst@uibk.ac.at)
References
1. Amort, Ch.: Hochaktive Iminchelat-Metallkomplexe (Fe, Co, Ni...) mit Pyrrolsubstituenten zur Polymerisation von Olefinen. PhD Dissertation, University of Innsbruck, Austria 2000.
Ni(1) 4a 1.1039(1) 0.90339(5) 0.23252(4) 0.0516(7) 0.0275(5) 0.0320(6) –0.0003(5) –0.0042(6) –0.0009(4) Br(1) 4a 0.88604(9) 0.86370(5) 0.24611(4) 0.0547(5) 0.0564(5) 0.0632(6) –0.0149(5) –0.0114(5) –0.0011(4) Br(2) 4a 1.2530(1) 0.83631(5) 0.17600(4) 0.0916(8) 0.0668(6) 0.0641(6) 0.0160(7) 0.0231(6) –0.0113(5) N(1) 4a 1.0867(5) 1.0199(3) 0.2187(3) 0.024(4) 0.033(4) 0.028(4) –0.001(3) –0.001(3) 0.012(3) N(2) 4a 1.1686(5) 0.9388(4) 0.3125(2) 0.039(4) 0.030(4) 0.023(4) 0.004(3) –0.005(3) 0.006(3) C(1) 4a 1.1133(8) 1.0580(4) 0.2650(3) 0.040(5) 0.031(5) 0.025(5) 0.000(4) –0.006(4) –0.002(4)
C(2) 4a 1.1583(7) 1.0126(4) 0.3182(3) 0.029(5) 0.028(5) 0.033(5) 0.002(4) 0.002(4) 0.003(4)
C(3) 4a 1.1799(8) 1.0729(4) 0.3652(3) 0.060(6) 0.026(5) 0.025(5) 0.006(4) –0.012(4) –0.007(4) C(4) 4a 1.0393(9) 1.1009(5) 0.3802(4) 0.082(8) 0.043(6) 0.041(5) 0.019(5) 0.003(5) –0.007(5) C(5) 4a 0.9942(9) 1.1483(5) 0.3246(4) 0.089(8) 0.036(6) 0.061(6) 0.023(5) –0.029(6) –0.012(5) C(6) 4a 1.1132(9) 1.1409(4) 0.2827(3) 0.065(6) 0.034(5) 0.036(5) 0.006(5) –0.011(5) 0.001(4) C(7) 4a 1.2314(9) 1.1423(4) 0.3258(3) 0.083(7) 0.028(5) 0.039(5) –0.001(5) –0.029(6) 0.000(5) C(8) 4a 1.261(1) 1.0506(4) 0.4193(3) 0.076(6) 0.046(5) 0.035(5) 0.001(6) –0.012(5) –0.012(4) C(9) 4a 1.365(1) 1.1242(5) 0.2963(4) 0.080(8) 0.050(6) 0.059(6) –0.025(6) –0.015(6) 0.012(5) C(10) 4a 1.245(1) 1.2182(4) 0.3607(4) 0.121(9) 0.037(5) 0.069(7) –0.009(6) –0.043(7) –0.006(5) C(11) 4a 1.0473(8) 1.0551(4) 0.1636(3) 0.045(6) 0.022(4) 0.025(5) –0.001(4) –0.007(5) 0.001(4) C(12) 4a 1.1433(8) 1.0629(4) 0.1203(3) 0.058(7) 0.035(5) 0.021(5) –0.005(4) –0.012(5) –0.001(4) C(13) 4a 1.104(1) 1.0880(5) 0.0655(3) 0.063(7) 0.052(6) 0.042(6) –0.016(5) 0.002(6) 0.005(5) C(14) 4a 0.974(1) 1.1052(5) 0.0535(4) 0.093(9) 0.055(6) 0.032(6) –0.010(6) –0.029(6) 0.009(5) C(15) 4a 0.8823(9) 1.0982(5) 0.0968(4) 0.046(6) 0.060(6) 0.057(6) 0.005(5) –0.014(6) 0.017(5) C(16) 4a 0.9146(9) 1.0726(4) 0.1534(3) 0.049(7) 0.036(5) 0.028(5) 0.004(4) –0.005(5) 0.004(4) C(17) 4a 1.2896(8) 1.0507(5) 0.1310(3) 0.051(7) 0.043(5) 0.032(5) –0.004(5) –0.003(4) 0.002(4) C(18) 4a 1.3566(9) 1.0010(5) 0.0846(4) 0.060(7) 0.087(7) 0.054(6) 0.000(6) 0.011(5) 0.007(5) C(19) 4a 1.3575(8) 1.1306(5) 0.1367(4) 0.049(6) 0.066(6) 0.072(6) –0.006(6) –0.008(5) 0.011(5) C(20) 4a 0.8060(8) 1.0664(5) 0.1979(3) 0.048(6) 0.053(6) 0.050(6) 0.010(5) –0.012(5) 0.003(4) C(21) 4a 0.6919(9) 1.0140(6) 0.1765(5) 0.044(6) 0.116(9) 0.089(8) –0.005(6) –0.015(6) 0.040(7) C(22) 4a 0.755(1) 1.1480(6) 0.2123(4) 0.101(8) 0.097(8) 0.065(7) 0.053(8) –0.002(6) 0.008(6) C(23) 4a 1.2143(8) 0.8873(4) 0.3587(3) 0.039(6) 0.032(5) 0.026(5) 0.001(4) –0.005(4) 0.003(4) C(24) 4a 1.3467(8) 0.8659(4) 0.3583(3) 0.059(7) 0.020(4) 0.036(5) –0.001(5) –0.018(5) 0.004(4) C(25) 4a 1.3921(9) 0.8154(5) 0.4014(4) 0.055(6) 0.044(6) 0.061(6) –0.005(5) –0.020(6) 0.002(5) C(26) 4a 1.307(1) 0.7858(5) 0.4431(4) 0.087(9) 0.035(5) 0.057(7) 0.005(6) –0.040(7) 0.018(5) C(27) 4a 1.177(1) 0.8075(5) 0.4422(3) 0.089(8) 0.048(6) 0.029(5) –0.010(6) –0.010(5) 0.008(5) C(28) 4a 1.1254(9) 0.8577(4) 0.3997(3) 0.062(7) 0.031(5) 0.029(5) –0.014(5) –0.014(5) 0.002(4) C(29) 4a 1.4502(8) 0.8929(4) 0.3139(4) 0.052(6) 0.040(5) 0.049(5) 0.011(5) –0.002(5) 0.002(5) C(30) 4a 1.5142(9) 0.8232(5) 0.2821(4) 0.078(7) 0.055(6) 0.071(6) 0.007(5) 0.006(5) –0.009(6) C(31) 4a 1.5581(9) 0.9433(5) 0.3423(4) 0.065(7) 0.058(6) 0.091(7) –0.004(5) –0.017(6) –0.011(6) C(32) 4a 0.9796(9) 0.8756(5) 0.3999(3) 0.059(7) 0.062(7) 0.031(5) –0.010(5) 0.005(5) 0.009(5) C(33) 4a 0.898(1) 0.7993(5) 0.3907(4) 0.068(7) 0.063(6) 0.075(6) –0.023(6) –0.012(6) 0.024(5) C(34) 4a 0.932(1) 0.9152(5) 0.4561(4) 0.068(8) 0.089(7) 0.061(6) –0.014(6) 0.014(5) 0.012(6)
C(35) 4a 1.479(2) 1.304(1) 0.4652(8) 0.31(3) 0.21(2) 0.15(2) –0.12(2) –0.08(2) –0.02(2)
C(36) 4a 1.523(3) 1.234(1) 0.471(1) 0.35(4) 0.05(1) 0.24(3) –0.13(2) –0.22(3) 0.08(2)
C(37) 4a 1.451(2) 1.205(1) 0.5297(9) 0.18(2) 0.11(1) 0.15(2) –0.04(1) –0.06(1) –0.03(1)
C(38) 4a 1.513(2) 1.131(2) 0.5396(8) 0.14(2) 0.21(2) 0.10(1) –0.08(2) 0.01(1) –0.04(2)
C(39) 4a 1.611(3) 1.090(2) 0.503(1) 0.27(3) 0.27(3) 0.13(2) –0.15(3) –0.08(2) 0.10(2)
C(40) 4a 1.661(3) 1.116(2) 0.447(2) 0.24(4) 0.16(2) 0.60(7) –0.03(2) –0.27(4) –0.01(4)
C(41) 4a 1.609(2) 1.183(2) 0.443(1) 0.11(2) 0.22(3) 0.20(2) –0.07(2) –0.00(2) –0.06(2)
Table 3.Atomic coordinates and displacement parameters (in Å2).
Atom Site x y z U11 U22 U33 U12 U13 U23
H(22A) 4a 0.6847 1.1443 0.2409 0.132
H(22B) 4a 0.7223 1.1725 0.1770 0.132
H(22C) 4a 0.8269 1.1791 0.2283 0.132
H(25) 4a 1.4818 0.8011 0.4021 0.064
H(26) 4a 1.3378 0.7510 0.4718 0.072
H(27) 4a 1.1195 0.7880 0.4713 0.066
H(29) 4a 1.4047 0.9253 0.2842 0.056
H(30A) 4a 1.5787 0.8423 0.2541 0.102
H(30B) 4a 1.4465 0.7937 0.2617 0.102
H(30C) 4a 1.5576 0.7898 0.3104 0.102
Table 2.Continued
.
Atom Site x y z Uiso
H(31A) 4a 1.6210 0.9596 0.3127 0.107
H(31B) 4a 1.6033 0.9131 0.3722 0.107
H(31C) 4a 1.5181 0.9891 0.3601 0.107
H(32) 4a 0.9609 0.9111 0.3667 0.060
H(33A) 4a 0.8048 0.8116 0.3910 0.103
H(33B) 4a 0.9180 0.7628 0.4220 0.103
H(33C) 4a 0.9217 0.7761 0.3534 0.103
H(34A) 4a 0.8376 0.9255 0.4532 0.109
H(34B) 4a 0.9786 0.9641 0.4616 0.109
H(34C) 4a 0.9482 0.8811 0.4893 0.109
Table 2.Continued
.
Atom Site x y z Uiso
2. Sheldrick, G. M.: SHELXS-97, Program for Crystal Structure Solution, University of Göttingen, Germany 1997.
3. Sheldrick, G. M.: SHELXL-97, Program for Crystal Structure Refine- ment, University of Göttingen, Germany 1997.
4. Sheldrick, G. M.: Program package SHELXTL Version 5.1, Bruker Ana- lytical X-Ray Instruments Inc, Madison (WI 53719), USA 1997.