Zeitschrift für Kristallographie - New Crystal Structures 212, 2 0 7 - 2 0 8
© by R. Oldenbourg Verlag, München 1997
207
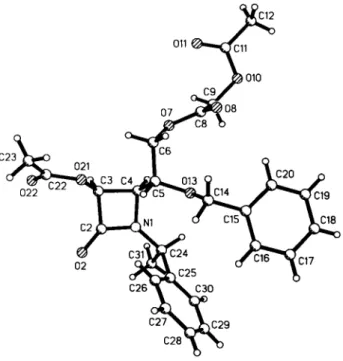
C r y s t a l s t r u c t u r e o f ( 3 5 , 4 / ^ , 1 7 ? , l 7 ^ ) - 3 - a c e t o x y - 4 - [ 2
, ,- a c e t o x y a c e t o x y - l
,- b e n z y l o x y - e t h y l ] - l - ( r - p h e n y l e t h y l ) - a z e t i d i n - 2 - o n e , C 2 6 H 2 9 N O 8
S. Henkel, Β. Krämer and V. Jäger
Universität Stuttgart, Institut für Organische Chemie, Pfaffenwaldring 55, D-70569 Stuttgart, Germany Received August 16, 1996, CSD-No. 402600
C2:
Source of material: The title compound (alternative name: (17?)- 2-0-aœtyl-5-acetoxy-acetoxy-3-anmno-4-0-benzyl-3-deoxy-W-( 1 '- phenylethyl)-L-xylono-l,3-lactam; see ref. 1) was prepared by [2+2]-cycloaddition of (2fl)-2-0-benzyl-glyceraldehyde-N-( 1 phenylethyl)imine (see refs. 2, 3) with acetoxyacetylchloiide in CH2Cl2/Et3N at 243 Κ (see ref. 1). The reaction gave a 92 : 8 mixture of cis-diastereomers, which were separated by crystal- lization. The structure represents the main diastereomer. The azetidin-2-one was crystallized from petroleum ether/ethyl acetate (70:30).
C26H29N08, orthorhombic, P2i2i2i (No. 19), a =8.429(1) Â,
¿7=11.135(1) Â, c =27.156(4) Â, V =2548.8 Â
3, Ζ =4, R(F) =0.050,
Table 1. Parameters used for the X-ray data collection
Crystal: colorless block, size 0.4 χ 0.5 χ 0.8 mm Wavelength: Mo Ka radiation (0.71073 A)
μ: 0.94 cm 1
Diffractometen Nicolet P3
Scan mode: Wyckoff
^measurement' 293 Κ
20max: 56°
3488 Criterion for /0:
I
a>2
σ(/0)N(param)rrfmej:
325Programs: SHELXS-86, SHELXL-93
Table 2. Final atomic coordinates and displacement parameters (in À2)
Atom Site
X y
ζ Ή »H(3) 4a 0.465(4) 0.622(3) 0.136(1) 0.045(8) H(4) 4a 0.605(4) 0.503(3) 0.084(1) 0.045(8) H(5) 4a 0.8607(3) 0.6715(2) 0.10080(9) 0.046 H(6A) 4a 0.6694(4) 0.7599(3) 0.0494(1) 0.060 H(6B) 4a 0.8255(4) 0.7373(3) 0.0191(1) 0.060 H(9A)
4a
0.6582(6) 0.4200(4) -0.0504(1) 0.095 H(9B)4a
0.5129(6) 0.5070(4) -0.0556(1) 0.095 H(12A)4a
0.6247(6) 0.4961(4) -0.2031(1) 0.116 H(12B)4a
0.6206(6) 0.6362(4) -0.2086(1) 0.116 H(12C)4a
0.4617(6) 0.5632(4) -0.2071(1) 0.116 H(14A)4a
1.1104(4) 0.5980(3) 0.0831(1) 0.061 H(14B)4a
1.0658(4) 0.6387(3) 0.0293(1) 0.061 H(16)4a
1.2599(5) 0.4154(4) 0.0946(1) 0.080 H(17)4a
1.4020(6) 0.2566(4) 0.0615(2) 0.101 H(18)4a
1.3891(6) 0.2172(4) -0.0216(2) 0.098 H(19)4a
1.2350(5) 0.3346(4) -0.0716(2) 0.089 H(20)4a
1.0966(4) 0.4938(3) -0.0395(1) 0.069 H(23A)4a
0.6436(6) 0.9651(3) 0.1413(1) 0.101 H(23B)4a
0.5718(6) 0.9887(3) 0.1937(1) 0.101 H(23C)4a
0.4651(6) 1.0023(3) 0.1468(1) 0.101 H(24)4a
0.8230(4) 0.3440(2) 0.1193(1) 0.050 H(26)4a
0.9793(4) 0.5712(3) 0.1815(1) 0.059 H(27)4a
1.2376(4) 0.5852(4) 0.2120(1) 0.076 H(28)4a
1.3866(5) 0.4154(5) 0.2236(1) 0.098 H(29)4a
1.2823(5) 0.2300(5) 0.2042(2) 0.101 H(30)4a
1.0313(5) 0.2158(4) 0.1713(2) 0.083 H(31A)4a
0.6073(4) 0.2783(3) 0.1670(2) 0.094 H(31B)4a
0.6998(4) 0.3111(3) 0.2152(2) 0.094 H(31C)4a
0.7616(4) 0.2076(3) 0.1811(2) 0.094208
C26H29N08 Table 3. Final atomic coordinates and displacement parameters (in A2)Atom Site X y ζ Un 1/22 Un Un Un f / 2 3
N(l) 4a 0.7242(3) 0.4886(2) 0.14852(8) 0.049(1) 0.039(1) 0.035(1) 0.001(1) 0.002(1) 0.0005(9) C(2) 4a 0.6462(4) 0.5581(3) 0.1818(1) 0.049(2) 0.043(2) 0.041(1) -0.004(1) 0.010(1) -0.001(1) 0(2) 4a 0.6421(3) 0.5573(2) 0.22627(7) 0.082(2) 0.064(1) 0.036(1) 0.004(1) 0.008(1) -0.002(1) C(3) 4a 0.5692(4) 0.6321(3) 0.1406(1) 0.037(1) 0.046(2) 0.043(1) 0.002(1) 0.005(1) -0.001(1) C(4) 4a 0.6703(3) 0.5557(2) 0.10469(9) 0.040(1) 0.039(1) 0.035(1) -0.000(1) -0.000(1) -0.002(1) C(5) 4a 0.8025(3) 0.6207(2) 0.07750(9) 0.044(1) 0.036(1) 0.035(1) -0.000(1) 0.004(1) -0.004(1) C(6) 4a 0.7385(4) 0.6982(3) 0.0361(1) 0.066(2) 0.047(2) 0.037(1) 0.005(2) 0.005(1) 0.002(1) 0(7) 4a 0.6512(3) 0.6235(2) 0.00220(7) 0.058(1) 0.073(1) 0.0359(9) 0.000(1) -0.002(1) 0.004(1) C(8) 4a 0.7188(4) 0.5951(3) -0.0410(1) 0.058(2) 0.063(2) 0.037(1) 0.010(2) -0.005(1) 0.007(1) 0(8) 4a 0.8355(3) 0.6387(3) -0.05728(8) 0.070(2) 0.113(2) 0.046(1) -0.011(2) 0.011(1) -0.010(1) C(9) 4a 0.6239(6) 0.4963(4) -0.0640(1) 0.114(4) 0.074(2) 0.051(2) -0.012(3) -0.012(2) 0.002(2) 0(10) 4a 0.6396(4) 0.4928(2) -0.11652(8) 0.096(2) 0.059(1) 0.053(1) 0.013(2) -0.014(1) -0.013(1) C ( l l ) 4a 0.5630(4) 0.5804(3) -0.1401(1) 0.063(2) 0.064(2) 0.057(2) 0.014(2) -0.012(2) -0.018(2) 0(11) 4a 0.5023(5) 0.6590(3) -0.1180(1) 0.159(3) 0.129(3) 0.087(2) 0.094(3) -0.025(2) -0.036(2) C(12) 4a 0.5679(6) 0.5679(4) -0.1945(1) 0.086(3) 0.096(3) 0.050(2) -0.010(3) -0.015(2) -0.009(2) 0(13) 4a 0.9060(2) 0.5294(2) 0.05942(6) 0.044(1) 0.0367(9) 0.0425(9) -0.0021(9) 0.0086(9) -0.0046(8) C(14) 4a 1.0654(4) 0.5714(3) 0.0520(1) 0.048(2) 0.048(2) 0.058(2) -0.006(2) 0.014(2) -0.008(1) C(15) 4a 1.1615(4) 0.4702(3) 0.0315(1) 0.041(2) 0.045(2) 0.053(2) -0.006(1) 0.014(1) -0.006(1) C(16) 4a 1.2541(5) 0.3996(4) 0.0610(1) 0.064(2) 0.075(2) 0.060(2) 0.007(2) 0.003(2) 0.003(2) C(17) 4a 1.3396(6) 0.3044(4) 0.0412(2) 0.079(3) 0.076(3) 0.096(3) 0.025(3) 0.003(3) 0.011(2) C(18) 4a 1.3319(6) 0.2809(4) -0.0084(2) 0.081(3) 0.068(3) 0.095(3) 0.020(2) 0.023(3) -0.018(2) C(19) 4a 1.2407(5) 0.3509(4) -0.0381(2) 0.080(3) 0.076(3) 0.066(2) 0.004(2) 0.020(2) -0.020(2) C(20) 4a 1.1571(4) 0.4455(3) -0.0187(1) 0.061(2) 0.061(2) 0.052(2) 0.004(2) 0.011(2) -0.004(2) CK21) 4a 0.6065(2) 0.7569(2) 0.14008(7) 0.050(1) 0.041(1) 0.049(1) 0.005(1) 0.008(1) -0.0030(9) C(22) 4a 0.5046(5) 0.8284(3) 0.1651(1) 0.063(2) 0.054(2) 0.048(2) 0.009(2) 0.008(2) -0.004(2) 0(22) 4a 0.3953(4) 0.7902(3) 0.1872(1) 0.096(2) 0.067(2) 0.114(2) 0.005(2) 0.062(2) -0.010(2) C(23) 4a 0.5504(6) 0.9577(3) 0.1614(1) 0.093(3) 0.046(2) 0.063(2) 0.005(2) 0.002(2) -0.006(2) C(24) 4a 0.8108(4) 0.3759(2) 0.1527(1) 0.051(2) 0.033(1) 0.041(1) -0.001(1) 0.002(1) 0.000(1) C(25) 4a 0.9772(4) 0.3917(3) 0.1737(1) 0.046(2) 0.045(2) 0.040(1) -0.000(1) 0.005(1) 0.004(1) C(26) 4a 1.0398(4) 0.5021(3) 0.1857(1) 0.054(2) 0.053(2) 0.041(2) -0.007(2) 0.004(1) 0.002(1) C(27) 4a 1.1953(4) 0.5104(4) 0.2043(1) 0.055(2) 0.086(3) 0.049(2) -0.023(2) 0.008(2) -0.003(2) C(28) 4a 1.2842(5) 0.4095(5) 0.2110(1) 0.049(2) 0.133(4) 0.062(2) 0.001(3) -0.002(2) 0.019(3) C(29) 4a 1.2224(5) 0.2991(5) 0.1992(2) 0.062(2) 0.094(3) 0.097(3) 0.020(3) -0.004(2) 0.023(3) C(30) 4a 1.0710(5) 0.2907(4) 0.1801(2) 0.059(2) 0.059(2) 0.090(3) 0.009(2) 0.000(2) 0.009(2) C(31) 4a 0.7104(4) 0.2846(3) 0.1817(2) 0.061(2) 0.045(2) 0.082(2) -0.011(2) 0.007(2) 0.012(2)
Acknowledgment. We gratefully acknowledge the support by the Fonds der Chemischen Industrie.
References
1. Krämer, Β. Franz, T.; Picasso, S.; Pnischke, P.; Jäger, V: Glycono-1,3- lactams, Xylo Series: Stereoselctive Access by [2+2]Cycloaddition, Exploratory Transformations, and Discovery of a New, Highly Selec- tive Inhibitor of Glucoamylases. Synlett. In print.
2. Veith, U.; Leurs, S.; Jäger, V.: Auxiliary-controlled diastereoselection by iV-( 1-phenylethy 1) in Grignard additions to 2-O-benzylglyceraldehyde imines. J. Chem. Soc. Chem. Commun. (19%) 329-330.
3. Franz, T.: Stereoselektive Synthesen von 3-Amino-l,2-diolen, Isoxa- zolinen und ß-Lactamen aus 2-O-Benzylglycerinaldehyd - Schlüssel- strukturen zum Aufbau von Natur und Wirkstoffen. Dissertation, University of Würzburg, Germany 1992.
4. Sheldrick, G. M.: SHELXS-86. Program for the solution of crystal structures. University of Göttingen, Germany 1986.
5. Sheldrick, G. M.: SHELXL-93, a program for refining crystal structures.
University of Göttingen, Germany 1993.