Ζ. Kristallogr. N C S 2 1 5 ( 2 0 0 0 ) 1 3 3 - 1 3 4
© b y O l d e n b o u r g W i s s e n s c h a f t s v e r l a g , M ü n c h e n
1 3 3
Crystal structure of (+)-(4S,5R,2'R,4'S,5'R,l"S)-5-(2'-acetoxy- l"-benzyloxy-ethyl)-3-(5'-acetoxy-2'-methyl-l',3'-dioxan-4'-yl)- 4-(ter^butoxycarbonylamino)-4,5-dihydro-isoxazole, C26H36N2O10
S. Henkel, Μ. Fengler-Veith and V. Jäger*
Universität Stuttgart, Institut für Organische Chemie, Pfaffenwaldring 55, D-70569 Stuttgart, Germany Received July 23, 1999, CCDC-No. 1267/235
C2:
Table 1. Data collection and handling.
Abstract
C 2 6 H 3 6 N 2 O 1 0 , m o n o c l i n i c , P 1 2 i l ( N o . 4 ) , α = 5 . 1 5 5 ( 1 ) Ä , b = 2 0 . 1 4 6 ( 6 ) Ä , с = 1 3 . 4 9 4 ( 2 ) Ä , β = 9 3 . 2 1 ( 2 ) ° , V = 1 3 9 9 . 2 Ä3, Z= 4 , Rgt(F) = 0 . 0 7 3 , wR(F2) = 0 . 1 9 1 , Τ = 2 9 3 К .
Source of material
T h e title c o m p o u n d [ 1 , 2 ] h a s b e e n o b t a i n e d b y d i a c e t y l a t i o n o f {AS,5R,2'RA'S,5'R, l " S ) - 5 - ( 1 " - O - b e n z y l - 1 " , 2 " - d i h y d r o x y e t h y l ) - 3 ( 5 ' - h y d r o x y - 2 ' - m e t h y l - 1 ' , 3 ' - d i o x a n ^ 4 ' - y l ) - 4 , 5 - d i h y d r o - i s o x a z o l e - 4 - c a r b o x a m i d e [ 1 , 2 ] w i t h a c e t i c a n h y d r i d e a n d c a t a l y t i c a m o u n t s o f D M A P i n p y r i d i n e , f o l l o w e d b y H o f m a n n d e g r a d a t i o n w i t h l e a d t e t r a a c e t a t e i n f m - b u t a n o l at 3 5 3 К - 3 5 8 К [ 3 , 4 ] . P u r i f i c a - t i o n b y f l a s h c h r o m a t o g r a p h y o n s i l i c a a n d c r y s t a l l i z a t i o n f r o m E t O A c / p e t r o l e t h e r g a v e t h e i s o x a z o l e in 8 4 % o v e r a l l y i e l d , ( m p 3 6 5 К - 3 6 8 Κ , [ α ] π = + 8 6 . 4 , с = 1 . 5 6 , E t O H ) .
Discussion
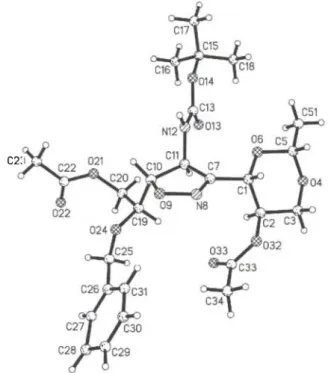
T h e c e l l p l o t o f t h e structure s h o w s a n a n t i p a r a l l e l o r i e n t a t i o n o f the m o l e c u l e s a l o n g t h e α - a x i s . A l o n g t h e α - a x i s , w e a l s o o b s e r v e a s t a c k i n g o r i e n t a t i o n o f t h e b e n z y l g r o u p s a n d l i k e w i s e o f t h e d i o x o l a n e m o i e t i e s .
Crystal: colourless block, size 0.6 χ 0.65 χ 0.7 mm Wavelength: M o Ka radiation (0.71073 Ä)
μ: 0.98 cnT1
Diffractometer, scan mode: Nicolet P3, Wyckoff
28max: 55°
WrW)measured, N(hkl)mique: 3643, 3304 Criterion for /0bs, N(hkl)gt: U s > 2 af/cbs), 2525 N(param) refined: 343
Programs: SHELXS-86 [5], SHELXL-93 [6]
Table 2. Atomic coordinates and displacement parameters (in Ä2).
Atom Site X У ζ Uiso
H ( l ) 2 a 1.0128(8) 0.6099(3) 1.0061(3) 0.059 H(2) 2 a 1.4453(9) 0.5342(3) 0.9436(4) 0.062 H(3A) 2 a 1.330(1) 0.5607(4) 1.1430(4) 0.081 H(3B) 2 a 1.575(1) 0.5217(4) 1.1098(4) 0.081 H(5) 2 a 1.304(1) 0.6788(3) 1.1080(4) 0.075 H(10) 2 a 0.9062(7) 0.6119(2) 0.6337(3) 0.051 H ( l l ) 2 a 1.3300(7) 0.6077(2) 0.7605(3) 0.046 H(12) 2 a 0.9710(6) 0.7102(2) 0.7500(3) 0.051 H(16A) la 1.481(1) 0.8391(4) 0.5845(5) 0.107 H(16B) 2a 1.699(1) 0.8014(4) 0.6476(5) 0.107 H(16C) 2 a 1.713(1) 0.8787(4) 0.6360(5) 0.107 H(17A) 2 a 1.162(1) 0.9082(3) 0.6663(8) 0.139 H(17B) 2 a 1.379(1) 0.9499(3) 0.7236(8) 0.139 H(17C) 2 a 1.168(1) 0.9132(3) 0.7824(8) 0.139 H(18A) 2 a 1.729(1) 0.8154(4) 0.8331(5) 0.109 H(18B) 2 a 1.515(1) 0.8563(4) 0.8845(5) 0.109 H(18C) 2 a 1.726(1) 0.8931(4) 0.8257(5) 0.109 H(19) 2 a 1.2439(9) 0.5051(3) 0.6903(4) 0.060 H(20A) 2 a 1.415(1) 0.5858(4) 0.5881(4) 0.081 H(20B) 2a 1.391(1) 0.5178(4) 0.5307(4) 0.081 H(23A) 2a 0.822(2) 0.6265(5) 0.3653(5) 0.138 H(23B) 2 a 0.793(2) 0.5611(5) 0.3026(5) 0.138 H(23C) 2 a 0.989(2) 0.6161(5) 0.2728(5) 0.138 H(25A) 2a 1.231(2) 0.4155(5) 0.6098(9) 0.141 H(25B) 2 a 1.081(2) 0.4177(5) 0.5051(9) 0.141 H(27) 2 a 1.032(2) 0.3031(6) 0.5164(7) 0.127 H(28) 2a 0.727(3) 0.2076(5) 0.551(1) 0.182 H(29) 2a 0.452(3) 0.2319(6) 0.685(1) 0.158 H(30) 2a 0.475(2) 0.3295(5) 0.7545(9) 0.145 H(31) 2a 0.719(2) 0.4117(6) 0.7093(8) 0.136 H(34A) 2a 0.846(1) 0.4021(4) 1.0129(6) 0.128 H(34B) 2 a 1.011(1) 0.3462(4) 0.9659(6) 0.128 H(34C) 2a 0.817(1) 0.3886(4) 0.8984(6) 0.128 H(51A) 2 a 1.496(2) 0.7697(4) 1.0347(6) 0.129 H(51B) 2a 1.731(2) 0.7252(4) 1.0070(6) 0.129 H(51C) 2a 1.685(2) 0.7404(4) 1.1187(6) 0.129
* C o r r e s p o n d e n c e a u t h o r (e-mail: j a g e r . i o c @ p o . u n i - s t u t t g a r t . d e )
134 C26H
36N
2Ol0
Table 3. Atomic coordinates and displacement parameters (in Ä2).
Atom Site X У г Uli t/22 t/зз U12 Uli С/23
C(l) 2a 1.1523(8) 0.6042(3) 0.9604(3) 0.042(2) 0.063(3) 0.045(2) 0.006(2) 0.012(2) -0.004(2) C(2) 2a 1.3191(9) 0.5442(3) 0.9932(4) 0.043(2) 0.058(3) 0.055(3) 0.001(2) 0.007(2) 0.009(2) C(3) 2 a 1.456(1) 0.5578(4) 1.0924(4) 0.064(3) 0.080(4) 0.058(3) -0.001(3) -0.002(2) 0.011(3) 0(4) 2 a 1.5975(7) 0.6186(2) 1.0884(3) 0.057(2) 0.078(3) 0.054(2) -0.003(2) -0.004(2) 0.001(2) C(5) 2 a 1.435(1) 0.6722(3) 1.0592(4) 0.063(3) 0.074(4) 0.051(3) 0.001(3) 0.007(2) -0.019(3) 0(6) 2a 1.3127(7) 0.6611(2) 0.9643(2) 0.060(2) 0.056(2) 0.050(2) -0.002(2) -0.002(2) -0.004(2) C(7) 2a 1.0362(7) 0.5959(2) 0.8571(3) 0.035(2) 0.041(2) 0.053(2) 0.004(2) 0.006(2) 0.001(2) N(8) 2 a 0.8300(7) 0.5613(2) 0.8431(3) 0.036(2) 0.060(2) 0.058(2) -0.003(2) 0.010(2) 0.001(2) 0(9) 2 a 0.7700(5) 0.5547(2) 0.7412(3) 0.030(1) 0.058(2) 0.070(2) -0.006(1) 0.005(1) -0.007(2) C(10) 2 a 0.9778(7) 0.5818(2) 0.6854(3) 0.031(2) 0.048(2) 0.048(2) -0.002(2) -0.001(2) 0.001(2) C ( l l ) 2 a 1.1476(7) 0.6211(2) 0.7628(3) 0.025(2) 0.044(2) 0.046(2) -0.001(2) 0.001(1) -0.001(2) N(12) 2 a 1.1234(6) 0.6927(2) 0.7530(3) 0.023(1) 0.044(2) 0.060(2) 0.002(1) 0.001(1) 0.002(2) C(13) 2 a 1.3334(8) 0.7327(2) 0.7483(4) 0.034(2) 0.047(2) 0.059(3) -0.002(2) 0.004(2) -0.001(2) 0(13) 2a 1.5554(6) 0.7125(2) 0.7477(3) 0.026(1) 0.060(2) 0.100(3) -0.001(1) 0.007(2) -0.002(2) 0(14) 2 a 1.2566(6) 0.7957(2) 0.7435(3) 0.033(2) 0.048(2) 0.100(3) -0.005(1) 0.003(2) 0.002(2) C(15) 2a 1.4451(9) 0.8499(3) 0.7341(5) 0.043(2) 0.040(2) 0.092(4) -0.010(2) 0.004(2) 0.006(2) C(16) 2 a 1.599(1) 0.8415(4) 0.6421(5) 0.066(3) 0.078(4) 0.071(3) -0.023(3) 0.000(3) 0.004(3) C(17) 2 a 1.273(1) 0.9109(3) 0.7258(8) 0.061(3) 0.055(4) 0.160(7) -0.006(3) -0.001(4) 0.008(4) C(18) 2 a 1.620(1) 0.8541(4) 0.8280(5) 0.063(3) 0.081(4) 0.075(4) -0.020(3) 0.008(3) -0.009(3) C(19) 2a 1.1301(9) 0.5261(3) 0.6387(4) 0.040(2) 0.057(3) 0.051(2) 0.010(2) -0.004(2) -0.003(2) C(20) 2 a 1.293(1) 0.5534(4) 0.5596(4) 0.056(3) 0.095(4) 0.051(3) 0.021(3) 0.004(2) 0.005(3) 0(21) 2 a 1.1226(9) 0.5845(3) 0.4834(3) 0.092(3) 0.108(4) 0.049(2) 0.047(3) 0.007(2) 0.010(2) C(22) 2a 1.119(1) 0.5629(3) 0.3915(4) 0.059(3) 0.072(4) 0.058(3) -0.004(3) 0.003(2) -0.007(3) 0(22) 2 a 1.265(1) 0.5213(4) 0.3666(4) 0.116(4) 0.172(7) 0.088(4) 0.051(5) -0.005(3) -0.040(4) C(23) 2 a 0.913(2) 0.5944(5) 0.3274(5) 0.100(5) 0.109(6) 0.065(4) 0.010(5) -0.015(3) 0.002(4) 0(24) 2 a 0.9530(8) 0.4788(2) 0.5997(4) 0.059(2) 0.065(3) 0.089(3) -0.004(2) -0.004(2) -0.030(2) C(25) 2a 1.060(2) 0.4199(5) 0.5760(9) 0.121(7) 0.080(5) 0.156(9) -0.014(5) 0.036(6) -0.023(5) C(26) 2a 0.881(2) 0.3631(4) 0.6080(7) 0.082(4) 0.071(5) 0.108(6) -0.001(4) -0.017(4) -0.016(4) C(27) 2 a 0.906(2) 0.3076(6) 0.5627(7) 0.102(6) 0.122(8) 0.094(5) 0.015(6) 0.004(5) 0.014(5) C(28) 2 a 0.728(3) 0.2479(5) 0.584(1) 0.22(1) 0.064(5) 0.16(1) 0.025(7) -0.08(1) -0.006(6) C(29) 2 a 0.567(3) 0.2634(6) 0.663(1) 0.15(1) 0.083(7) 0.16(1) -0.035(7) 0.012(8) 0.018(7) C(30) 2 a 0.581(2) 0.3218(5) 0.7022(9) 0.126(8) 0.092(6) 0.144(8) -0.034(6) 0.003(6) 0.006(6) C(31) 2 a 0.727(2) 0.3711(6) 0.6770(8) 0.127(7) 0.104(7) 0.108(7) -0.033(6) -0.014(6) 0.030(5) 0(32) 2 a 1.1483(7) 0.4886(2) 1.0045(3) 0.058(2) 0.069(2) 0.060(2) -0.008(2) 0.009(2) 0.016(2) C(33) 2 a 1.148(1) 0.4385(3) 0.9393(5) 0.051(3) 0.061(3) 0.078(4) -0.003(2) -0.010(3) 0.002(3) 0(33) 2 a 1.297(1) 0.4368(3) 0.8733(5) 0.070(3) 0.103(4) 0.131(4) -0.022(3) 0.033(3) -0.046(3) C(34) 2a 0.937(1) 0.3895(4) 0.9556(6) 0.067(4) 0.080(4) 0.108(5) -0.016(3) -0.012(3) 0.014(4) C(51) 2a 1.601(2) 0.7322(4) 1.0545(6) 0.094(5) 0.078(4) 0.084(4) -0.008(4) -0.020(4) -0.020(4)
Acknowledgments. Financial support by Fonds der Chemischen Industrie is gratefully stated. We also thank Dr. Wolfgang Frey for help with the prepara- tion of the files.
References
1. Fengler-Veith, M.: Stereoselektive 1,3-dipolare Nitriloxid-Cyclo- additionen unter Normal- und Hochdruck - optisch aktive 4-Amino- isoxazoline durch Abbau bicyclischer Lactone. Dissertation, Universität Stuttgart, Germany 1996.
2. Jäger, V.; Müller, R.; Leibold, Т.; Hein, M.; Schwarz, M.; Fengler, M.;
Jaroskova, L.; Pätzel, M.; LeRoy, P.-Y.: Synthesis of Glycosidase In- hibiting Iminopolyols via Isoxazolines. Bull. Soc. Chim. Belg. 103 (1994) 491-507.
3. Baumgarten, Η. E.; Smith, H. L.; Staklis, Α.: Reactions of Amines. XVIII.
The Oxidative Rearrangement of Amides with Lead Tetraacetate. J. Org.
Chem. 40(1975)3554-3561.
4. Burgess, К.; Ho, K.-K.: Asymmetric Syntheses of All Four Stereoisomers of 2,3-Methanomethionine. J. Org. Chem. 57 (1992) 5931-5936.
5. Sheldrick, G. M.: Phase Annealing in SHELX-90: Direct Methods for Larger Structures. Acta Crystallogr. A46 (1990) 467-473.
6. Sheldrick, G. M.: SHELXL-93. Program for the Refinement of Crystal Structures. University of Göttingen, Germany 1993.