Zeitschrift f ü r Kristallographie - N e w Crystal Structures 2 1 2 , 2 2 3 - 2 2 5
© by R. O l d e n b o u r g Verlag, M ü n c h e n 1997
223
Crystal structure of (45,5/?,l / 5)-5-benzyloxy- J /V-(l , -phenylethyl)-4-(2 / thienyl)-tetrahydro-l,3-oxazin-2-one, C23H23NO3S
S. Henkel, Ν. Meunier and V. Jäger
Universität Stuttgart, Institut für Organische Chemie, Pfaffenwaldring 55, D-70569 Stuttgart, Germany Received September 9, 1996. CSD-No. 402624
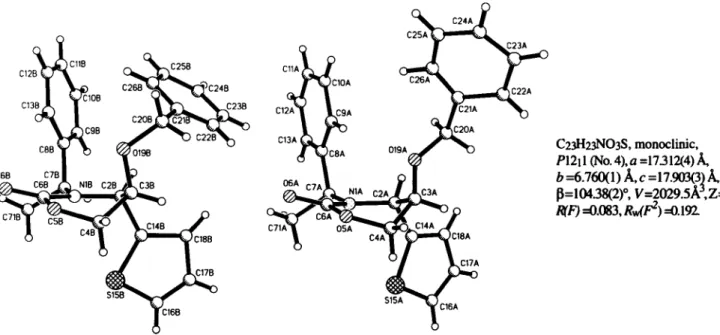
C23H23N03S, monoclinic, P\2\ 1 (No. 4), a =17312(4) Â, b = 6 . 7 6 0 ( 1 ) Â , c =17.903(3) A, ß = 1 0 4 3 8 ( 2 )0, V = 2 0 2 9 . 5 Â , Ζ = 4 , R(F) =0.083, RvJF2) =0.191
Source of material: The title compound (see réf. 1) was prepared by cyclization with triphosgene (see refs. 2,3) of the correspond- ing 3-amino-l,2-diol. The latter was obtained by addition of 2-thienyllithium to
(-)-(2R,1
'S)-0-benzy\-N-(1 '-phenylethyl) glycerinaldimine (see refs. 4,5).
In the crystal the tide compound is present as a 1:1 -mixture of two conformers A and B. In both, the tetrahydrooxazinone ring adopts a half-chair
5H6 (systematic ring numbering). The major dif- ference is that the phenyl group (in the O-benzyl part) of A is oriented
anti(torsional angle C3A-019A-C20A-C21A -155.67 degrees), in Β a
gauchearrangement (73.80 degrees) prevails. In the crystal the spatial arrangement shows alternating linear strings each composed of one type only.
Table 1. Parameters used for the X-ray data collection
Table 2. Final atomic coordinates and displacement parameters (in Â2)
Crystal: colorless block, size 0.2 χ 0.35 χ 0.7 mm Wavelength: Mo Ka radiation (0.71073 Â)
μ: 1.83 cm"1
Diffractometer: Nicolet P3
Scan mode: Wyckoff
Τmeasurement'· 293 Κ
26max: 54°
N(hkl)unique'. 4794
Criterion for I0: ¡o >2 σ(/ο)
N(param)refine<n 506
Programs: SHELXS-86, SHELXL-93
Atom Site X y ζ t/i«.
H(2A) 2a 0.0668(4) 0.368(1) 0.2674(4) 0.060 H(3A) 2a 0.0229(6) 0.112(1) 0.3477(6) 0.091 H(4A1) 2a 0.1420(5) 0.092(1) 0.4403(5) 0.089 H(4A2) 2a 0.1219(5) -0.116(1) 0.4016(5) 0.089 H(7A) 2a 0.1686(4) 0.619(1) 0.2935(4) 0.059 H(71A) 2a 0.2909(5) 0.600(2) 0.3866(5) 0.108 H(71B) 2a 0.2987(5) 0.720(2) 0.3139(5) 0.108 H(71C) 2a 0.3298(5) 0.502(2) 0.3254(5) 0.108 H(9A) 2a 0.1619(5) 0.220(2) 0.1860(5) 0.107 H(10A) 2a 0.1494(7) 0.207(4) 0.0520(7) 0.163 H(11A) 2a 0.1832(9) 0.490(5) -0.0080(6) 0.198 H(12A) 2a 0.2198(8) 0.766(3) 0.0579(8) 0.159 H(13A) 2a 0.2318(5) 0.781(2) 0.1903(6) 0.115 H(16A) 2a 0.0595(7) 0.697(2) 0.5372(6) 0.103 H(17A) 2a -0.0524(7) 0.739(2) 0.4302(8) 0.121 H(18A) 2a -0.0394(4) 0.565(1) 0.3153(5) 0.072 H(20A) 2a -0.0088(9) 0.136(2) 0.180(1) 0.222 H(20B) 2a -0.0539(9) 0.059(2) 0.241(1) 0.222 H(22A) 2a -0.1400(8) -0.225(3) 0.2048(6) 0.134 H(23A) 2a -0.1690(7) -0.526(2) 0.1342(7) 0.122 H(24A) 2a -0.0839(8) -0.613(2) 0.0643(8) 0.138 H(25A) 2a 0.021(1) -0.433(3) 0.0503(8) 0.161 H(26A) 2a 0.0487(7) -0.171(2) 0.1213(7) 0.115 H(2B) 2a 0.5754(4) 0.173(1) 0.2808(4) 0.053 H(3B) 2a 0.5339(6) -0.092(1) 0.3576(5) 0.079 H(4B1) 2a 0.6560(6) -0.104(1) 0.4505(5) 0.087
224
C23H23NO3STable 2. (Continued)
Atom Site
Table 2. (Continued)
Atom Site H(4B2) 2a 0.6352(6) -0.313(1) 0.4124(5) 0.087
H(7B) la 0.6772(4) 0.423(1) 0.3099(4) 0.054 H(71D) la 0.8051(5) 0.400(1) 0.3897(5) 0.093 H(71E) la 0.8010(5) 0.536(1) 0.3178(5) 0.093 H(71F) la 0.8354(5) 0.321(1) 0.3197(5) 0.093 H(9B) la 0.6623(5) 0.609(2) 0.1977(5) 0.081 H(10B) la 0.6405(6) 0.625(2) 0.0657(5) 0.110 H(11B) la 0.6596(6) 0.353(2) -0.0034(5) 0.104 H(12B) la 0.7014(6) 0.063(2) 0.0596(5) 0.096 H(13B) la 0.7235(5) 0.042(2) 0.1921(5) 0.079
H(16B) la 0.6047(5) 0.500(1) 0.5578(4) 0.068 H(17B) la 0.4772(5) 0.518(1) 0.4782(5) 0.077 H(18B) la 0.4595(3) 0.3422(8) 0.3459(3) 0.030 H(20C) la 0.4642(7) -0.266(2) 0.2576(8) 0.135 H(20D) la 0.5000(7) -0.333(2) 0.1895(8) 0.135 H(22B) la 0.3838(6) 0.027(3) 0.2369(7) 0.127 H(23B) la 0.3410(8) 0.318(3) 0.1735(9) 0.144 H(24B) la 0.4005(9) 0.423(3) 0.0789(8) 0.155 H(25B) la 0.4981(7) 0.231(3) 0.0467(7) 0.143 H(26B) la 0.5408(6) -0.049(3) 0.1121(6) 0.115
Table 3. Final atomic coordinates and displacement parameters (in Â2)
Atom Site χ y ζ Un U22 t/33 U12 Un U73 N(1A) la 0.1818(3) 0.3362(9) 0.3218(3) 0.057(3) 0.042(3) 0.047(3) 0.008(3) 0.017(3) -0.001(3) C(2A) la 0.0960(4) 0.327(1) 0.3193(4) 0.052(4) 0.040(4) 0.054(4) 0.000(4) 0.004(3) 0.002(4) C(3A) la 0.0723(6) 0.111(1) 0.3302(6) 0.071(6) 0.045(5) 0.108(7) -0.006(4) 0.015(5) -0.008(5) C(4A) la 0.1366(5) 0.019(1) 0.3927(5) 0.080(6) 0.039(5) 0.105(7) -0.002(5) 0.028(5) 0.017(5) CK5A) la 0.2107(3) 0.0185(9) 0.3719(3) 0.077(4) 0.050(3) 0.077(4) 0.017(3) 0.026(3) 0.016(3) C(6A) la 0.2317(5) 0.178(1) 0.3371(4) 0.063(5) 0.049(5) 0.051(4) 0.011(4) 0.017(4) 0.001(4) 0(6A) la 0.2965(4) 0.175(1) 0.3224(4) 0.089(4) 0.068(4) 0.083(4) 0.031(4) 0.039(3) 0.016(3) C(7A) 2a 0.2067(4) 0.516(1) 0.2879(4) 0.059(4) 0.038(4) 0.054(4) 0.010(4) 0.021(3) 0.003(3) C(71A) 2a 0.2893(5) 0.592(2) 0.3327(5) 0.084(6) 0.065(6) 0.066(5) -0.019(5) 0.016(5) -0.011(5) C(8A) 2a 0.1993(4) 0.499(2) 0.2019(4) 0.045(4) 0.088(7) 0.053(4) 0.013(5) 0.018(3) 0.015(5) C(9A) la 0.1743(5) 0.331(2) 0.1606(5) 0.076(6) 0.13(1) 0.052(5) 0.016(7) 0.006(4) -0.027(7) C(10A) 2a 0.1666(7) 0.321(4) 0.0800(7) 0.093(8) 0.25(2) 0.061(7) 0.03(1) 0.010(6) -0.04(1) C(11A) 2a 0.1862(9) 0.492(5) 0.0446(6) 0.11(1) 0.35(3) 0.043(6) 0.05(2) 0.029(6) 0.04(1) C(12A) la 0.2085(8) 0.654(3) 0.0835(8) 0.10(1) 0.23(2) 0.078(9) 0.02(1) 0.032(8) 0.07(1) C(13A) 2a 0.2159(5) 0.664(2) 0.1635(6) 0.064(6) 0.14(1) 0.086(7) 0.010(7) 0.026(5) 0.050(8) C(14A) 2a 0.0720(4) 0.461(1) 0.3742(4) 0.056(4) 0.029(3) 0.054(4) -0.003(3) 0.018(3) -0.001(3) C(16A) 2a 0.0555(7) 0.640(2) 0.4890(6) 0.108(8) 0.082(7) 0.090(7) -0.041(7) 0.069(6) -0.041(6) S(15A) 2a 0.1267(1) 0.4971(4) 0.4676(1) 0.080(1) 0.071(2) 0.058(1) -0.015(1) 0.018(1) -0.005(1) C(17A) 2a -0.0081(7) 0.664(2) 0.4281(8) 0.079(7) 0.071(7) 0.18(1) -0.019(6) 0.075(8) -0.042(8) C(18A) 2a -0.0008(4) 0.564(1) 0.3619(5) 0.040(4) 0.057(5) 0.081(5) -0.007(4) 0.013(4) -0.004(4) 0(19A) la 0.0606(4) -0.007(1) 0.2677(4) 0.115(5) 0.054(4) 0.114(5) -0.001(4) -0.025(4) -0.026(4) C(20A) la -0.0135(9) 0.026(2) 0.214(1) 0.17(1) 0.081(9) 0.23(2) 0.05(1) -0.10(1) -0.06(1) C(21A) la -0.0369(5) -0.153(2) 0.1674(5) 0.066(5) 0.063(6) 0.071(5) 0.011(5) -0.013(4) -0.017(5) C(22A) la -0.1077(8) -0.269(3) 0.1738(6) 0.12(1) 0.15(1) 0.078(7) 0.02(1) 0.032(6) 0.010(8) C(23A) 2a -0.1253(7) -0.448(2) 0.1326(7) 0.072(7) 0.11(1) 0.110(9) -0.032(7) 0.002(6) 0.016(8) C(24A) la -0.0741(8) -0.494(2) 0.0911(8) 0.089(9) 0.10(1) 0.14(1) 0.010(9) -0.011(8) 0.024(9) C(25A) la -0.011(1) -0.393(3) 0.0827(8) 0.13(1) 0.14(1) 0.12(1) 0.04(1) 0.02(1) -0.03(1) C(26A) la 0.0021(7) -0.237(2) 0.1235(7) 0.080(7) 0.11(1) 0.096(8) 0.010(7) 0.024(6) -0.014(8) N(1B) la 0.6925(3) 0.1396(8) 0.3295(3) 0.053(3) 0.036(3) 0.048(3) 0.006(3) 0.022(3) 0.003(3) C(2B) 2a 0.6075(4) 0.128(1) 0.3309(4) 0.054(4) 0.039(4) 0.041(4) 0.000(3) 0.015(3) 0.000(3) C(3B) 2a 0.5844(6) -0.088(1) 0.3421(5) 0.087(6) 0.041(4) 0.081(6) -0.023(4) 0.040(5) -0.021(4) C(4B) la 0.6494(6) -0.178(1) 0.4029(5) 0.116(7) 0.035(4) 0.081(6) 0.000(5) 0.052(5) 0.005(5) C(5B) la 0.7224(4) -0.1772(8) 0.3798(3) 0.103(4) 0.034(3) 0.091(4) 0.008(3) 0.034(3) 0.006(3) C(6B) la 0.7431(5) -0.014(1) 0.3455(4) 0.071(5) 0.044(4) 0.061(4) 0.011(4) 0.021(4) -0.002(4) CK6B) la 0.8083(4) -0.016(1) 0.3311(4) 0.072(4) 0.060(4) 0.095(4) 0.020(3) 0.033(3) 0.001(4) C(7B) 2a 0.7136(4) 0.324(1) 0.2976(4) 0.052(4) 0.034(3) 0.055(4) 0.008(3) 0.024(3) 0.001(3) C(71B) 2a 0.7963(5) 0.402(1) 0.3346(5) 0.061(5) 0.057(5) 0.064(5) -0.008(4) 0.009(4) -0.001(4) C(8B) 2a 0.6965(4) 0.325(1) 0.2101(4) 0.044(4) 0.052(4) 0.048(4) -0.006(4) 0.016(3) 0.004(4) C(9B) la 0.6708(5) 0.497(2) 0.1705(5) 0.068(5) 0.073(6) 0.065(5) 0.009(5) 0.023(4) 0.007(5) C(10B) la 0.6574(6) 0.506(2) 0.0911(5) 0.087(7) 0.13(1) 0.060(5) 0.027(8) 0.021(5) 0.027(7) C(11B) 2a 0.6687(6) 0.346(2) 0.0500(5) 0.076(6) 0.14(1) 0.051(5) 0.022(7) 0.023(4) 0.007(7) C(12B) la 0.6935(6) 0.174(2) 0.0876(5) 0.081(6) 0.102(8) 0.067(6) 0.003(6) 0.035(5) -0.024(6) C(13B) la 0.7071(5) 0.161(2) 0.1673(5) 0.068(5) 0.073(6) 0.063(5) -0.001(5) 0.028(4) -0.008(5) C(14B) la 0.5883(4) 0.2582(9) 0.3926(4) 0.054(4) 0.029(3) 0.044(4) -0.004(3) 0.016(3) 0.002(3) S(15B) la 0.6558(1) 0.3101(3) 0.4752(1) 0.090(2) 0.058(1) 0.061(1) 0.006(1) 0.024(1) -0.001(1) C(16B) la 0.5910(5) 0.440(1) 0.5097(4) 0.087(6) 0.044(4) 0.046(4) -0.013(4) 0.029(4) -0.008(3) C(17B) 2a 0.5181(5) 0.451(1) 0.4637(5) 0.066(5) 0.048(5) 0.087(6) 0.010(4) 0.036(5) -0.004(4) C(18B) la 0.5051(3) 0.3486(8) 0.3863(3) 0.034(3) 0.020(3) 0.027(2) 0.002(2) 0.019(2) -0.004(2) 0(19B) la 0.5785(4) -0.2020(9) 0.2740(4) 0.088(4) 0.058(4) 0.103(5) -0.013(4) 0.029(4) -0.036(4) C(20B) la 0.4995(7) -0.227(2) 0.2258(8) 0.103(9) 0.10(1) 0.14(1) -0.036(8) 0.043(8) -0.069(9) C(21B) la 0.4672(5) -0.042(2) 0.1816(6) 0.056(5) 0.106(9) 0.093(7) -0.030(6) 0.021(5) -0.061(7)
C23H23NO3S 225
Table 3. (Continued)
Atom Site U h U22 t/33 f/|2 Í/13 Í/23
C(22B) la C(23B) la Q 2 4 B ) 2a C(25B) la C(26B) la
0.4074(6) 0.3814(8) 0.4163(9) 0.4755(7) 0.5001(6)
0.070(3) 0.244(3) 0.305(3) 0.191(3) 0.024(3)
0.1985(7) 0.1609(9) 0.1049(8) 0.0862(7) 0.1246(6)
0.056(6) 0.072(7) 0.11(1) 0.067(7) 0.062(6)
0.18(2) 0.15(2) 0.15(1) 0.21(2) 0.15(1)
0.092(8) 0 . 1 2 ( 1 ) 0.11(1) 0.075(7) 0.076(7)
-0.019(8) 0.019(9) 0.01(1) -0.03(1) -0.004(8)
0.026(5) 0.003(7) -0.023(8) 0.006(6) 0.023(5)
-0.064(9) -0.06(1) -0.03(1) -0.03(1) -0.031(8)
Acknowledgments. We are grateful to the Fonds der Chemischen Industrie and the European Community (HCM network "Stereoselective Organic Syn- thesis") for financial support and to Deutscher Akademischer Auslandsdienst (DAAD) for awarding a doctoral fellowship to N. Meunier.
References
1. Meunier, Ν.: Stereoselektive nucleophile Additionen an optisch aktive Imine: Synthese von Hydroxyaminosäuren, Bestandteile von Natur- und Wirkstoffen. Dissertation, Universität Stuttgart Germany 1997.
2. Sicker, D.: A facile synthesis of 6-methoxy-2-oxo-2,3-dihydrobenzox- azole. Synthesis (1989) 875-876.
3. Peters, K.; Veith, U.; Jäger, V.: (-)-(4Λ,5Λ, 1 'S)-5-benzyloxy^-methyl-3- (l-phenyl-ethyl)-tetrahydro-l,3-oxazin-2-one. Z. Kristallogr. 212 (1997) 175-176.
4. Franz, T.; Hein, M.; Veith, U.; Jäger, V.; Peters, E.-M.; Peters, K.; von Schnering, H. G. : Einfache und variable Synthese optisch aktiver 1,2-Ami- noalkohole durch Grignard-Addition an Λ/,Ο-Dibenzylglyceraldimin und -lactaldimin. Angew. Chem. 106 (1994) 1308-1311; Angew. Chem. Int.
Ed. Engl. 3 3 (1994) 1298-1301.
5. Veith, U.; Leurs, S.; Jäger, V.: Auxiliary-controlled diastereoselection by iV-(l-phenylethyl) in Grignard additions to 2-O-benzylglyceraldehyde imines. J. Chem. Soc. Chem. Commun. (19%) 329-330.
6. Sheldrick, G. M.: SHELXS-86. Program for the solution of crystal struc- tures. University of Göttingen, Germany 1986.
7. Sheldrick, G. M.: SHELXL-93, a program for refining crystal structures.
University of Göttingen, Germany 1993.