Crystal structure of (3S,4S)-4,5-O-cyclohexylidene-4,5-dihydroxy-3- methylamino-pentanoic acid hydrochloride, (C 12 H 22 NO 4 )Cl
W. Frey, M. Henneböhle and V. Jäger*
Universität Stuttgart, Institut für Organische Chemie, Pfaffenwaldring 55, D-70569 Stuttgart, Germany Received February 27, 2004, accepted and available on-line April 27, 2004; CCDC no. 1267/1268
Abstract
C
12H
22ClNO
4, monoclinic, P12
11 (no. 4),
a = 6.2327(3) Å, b = 7.9360(3) Å, c = 14.1536(5) Å, b = 99.709(4)°, V = 690.1 Å
3, Z = 2,
R
gt(F) = 0.042, wR
ref(F
2) = 0.115, T = 293 K.
Source of material
The title compound has been obtained via (3S,1S ¢ )-3- [1,2-cyclo- hexylidenedioxyethyl]-2-methylisoxazolidine [1-4] in several steps, the first being hydrogenation with palladium on carbon.
The amino alcohol obtained was converted to the b -amino acid by N-protection with benzyl chlorocarbonate and then Zhao modifi- cation of the Jones oxidation using periodic acid/chromium(VI) oxide [5]. Deprotection with hydrogen/palladium on carbon in methanol, conversion to the hydrochloride salt and recrystalliza- tion from acetone/water afforded the title b -amino acid hydro- chloride in the form of colorless crystals (m.p. 451 K - 454 K, decomposition, [ a ]
20D= –32.3, c = 1.02, CH
3OH) [1,3,4].
Discussion
The absolute configuration was deduced from the absolute struc- ture determination by X-ray data indicated by the Flack parame- ter of x = –0.04(2). This confirms the steric course of reduction of an isoxazolidinium precursor, cf. [1-4]. The structure is built up by a network of intermolecular hydrogen bonds, with chloride ions acting as a triple acceptor. The hydrogen bonds are ² donated ² by N—H of the ammonium ion and O—H of the carboxylic acid.
The H4···Cl1 distance is 2.27 Å and the O4–H4···Cl1 angle is 164°. The H1A···Cl1 and H1B···Cl1 distances are 3.16 Å and 3.08 Å, respectively and the angles of N1–H1A···Cl1 and N1–H1B···Cl1 are 163° and 157°, respectively.
Z. Kristallogr. NCS
219(2004) 193–194 193
© by Oldenbourg Wissenschaftsverlag, München
Crystal: colorless plate, size 0.01 × 0.2 × 0.3 mm Wavelength: CuKaradiation (1.54178 Å)
m: 25.28 cm−1
Diffractometer, scan mode: Siemens P4,w
2qmax: 135.8°
N(hkl)measured,N(hkl)unique: 2324, 1977 Criterion forIobs,N(hkl)gt: Iobs> 2s(Iobs), 1820 N(param)refined: 165
Programs: SHELXS-97 [6], SHELXL-97 [7],
SHELXTL-XP [8]
Table 1.Data collection and handling.
H(1A) 2a 0.2953 1.3083 0.5758 0.046
H(1B) 2a 0.3005 1.1369 0.5406 0.046
H(1C) 2a −0.1372 1.2441 0.8132 0.061
H(1D) 2a 0.0579 1.1148 0.8260 0.061
H(2) 2a −0.0729 1.3059 0.6691 0.050
H(3) 2a 0.1008 1.0418 0.6542 0.043
H(4) 2a 0.4611 0.7519 0.6412 0.077
H(4A) 2a 0.5035 1.2062 0.7188 0.056
H(4B) 2a 0.3983 1.0872 0.7863 0.056
H(6A) 2a 0.0537 1.2856 0.4381 0.078
H(6B) 2a −0.0703 1.3265 0.5230 0.078
H(6C) 2a −0.0646 1.1414 0.4849 0.078
H(8A) 2a 0.4454 1.6435 0.8248 0.060
H(8B) 2a 0.5088 1.4688 0.8738 0.060
H(9A) 2a 0.3565 1.5478 1.0079 0.072
H(9B) 2a 0.5285 1.6843 0.9910 0.072
H(10A) 2a 0.2533 1.8611 0.9163 0.070
H(10B) 2a 0.2063 1.8144 1.0186 0.070
H(11A) 2a −0.0514 1.6158 0.9539 0.075
H(11B) 2a −0.1123 1.7888 0.9026 0.075
H(12A) 2a 0.0268 1.7043 0.7698 0.059
H(12B) 2a −0.1353 1.5643 0.7918 0.059
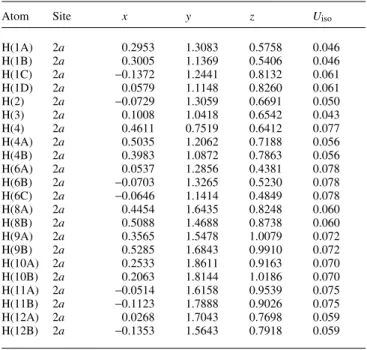
Table 2.Atomic coordinates and displacement parameters (in Å2)
.
Atom Site x y z Uiso
_____________
* Correspondence author (e-mail: jager.ioc@po.uni-stuttgart.de)
Cl(1) 2a 0.5589(1) 0.5094(1) 0.57557(6) 0.0500(4) 0.0393(5) 0.0573(5) −0.0044(4) 0.0223(3) −0.0003(4) O(1) 2a 0.1426(5) 1.3426(4) 0.8740(2) 0.063(2) 0.051(2) 0.043(1) −0.015(1) 0.011(1) 0.001(1) N(1) 2a 0.2191(4) 1.2117(4) 0.5667(2) 0.035(1) 0.036(2) 0.045(1) 0.002(1) 0.009(1) −0.006(1) C(1) 2a 0.0164(6) 1.2307(6) 0.8104(3) 0.054(2) 0.049(3) 0.053(2) −0.011(2) 0.021(2) −0.008(2) O(2) 2a 0.1967(4) 1.4247(4) 0.7275(2) 0.066(2) 0.037(1) 0.046(1) −0.005(1) 0.028(1) −0.006(1) C(2) 2a 0.0637(6) 1.2777(5) 0.7113(3) 0.040(2) 0.042(2) 0.046(2) 0.002(2) 0.013(2) −0.006(2) C(3) 2a 0.1865(5) 1.1459(5) 0.6630(2) 0.033(2) 0.036(2) 0.038(2) −0.001(1) 0.006(1) −0.002(1) O(3) 2a 0.7189(4) 0.9541(4) 0.6834(3) 0.037(1) 0.060(2) 0.108(2) 0.004(1) 0.008(2) −0.017(2) O(4) 2a 0.3926(4) 0.8319(3) 0.6576(2) 0.045(1) 0.039(2) 0.070(2) 0.002(1) 0.012(1) −0.008(1) C(4) 2a 0.4126(6) 1.1073(6) 0.7201(3) 0.045(2) 0.047(2) 0.045(2) 0.006(2) −0.000(2) −0.004(2) C(5) 2a 0.5275(5) 0.9583(4) 0.6848(2) 0.040(2) 0.034(2) 0.048(2) −0.001(2) 0.003(1) 0.003(1)
C(6) 2a 0.0165(7) 1.2442(6) 0.4970(3) 0.049(2) 0.057(3) 0.048(2) 0.010(2) 0.002(2) 0.005(2)
C(7) 2a 0.1833(5) 1.4873(5) 0.8213(2) 0.043(2) 0.042(2) 0.036(1) 0.000(2) 0.012(1) −0.002(2) C(8) 2a 0.4007(6) 1.5579(5) 0.8664(3) 0.040(2) 0.042(3) 0.069(2) −0.000(2) 0.012(2) −0.003(2) C(9) 2a 0.3888(7) 1.6352(7) 0.9645(3) 0.055(2) 0.064(3) 0.058(2) −0.012(2) −0.002(2) −0.006(2) C(10) 2a 0.2148(7) 1.7695(6) 0.9556(3) 0.066(3) 0.061(3) 0.049(2) −0.009(2) 0.017(2) −0.015(2) C(11) 2a −0.0056(7) 1.6987(7) 0.9110(3) 0.051(2) 0.071(3) 0.070(3) −0.001(2) 0.021(2) −0.023(2) C(12) 2a 0.0034(6) 1.6178(6) 0.8153(3) 0.043(2) 0.052(2) 0.052(2) 0.005(2) 0.006(2) −0.010(2) Table 3.Atomic coordinates and displacement parameters (in Å2).
Atom Site x y z U11 U22 U33 U12 U13 U23
Acknowledgment.We are grateful to the Fonds der Chemischen Industrie for financial support of this work.
References
1. Henneböhle, M.:N-Methylisoxazolinium-Salze – Neue Bausteine in der stereoselektiven Synthese von Aminopolyolen, Aminolactonen und Aminosäuren. Dissertation, Universität Stuttgart, Germany 2002.
2. Frey, W.; Henneböhle, M.; Jäger, V.: Crystal structure of (3S,1¢S)-2,2- dimethyl-3-[1,2-cyclohexylidenedioxyethyl]-tetrahydro-1,2-oxazolium tetrafluoroborate. Z. Kristallogr. NCS submitted.
3. Henneböhle, M.; Le Roy, P.-Y.; Hein, M.; Ehrler, R.; Jäger, V.:
Isoxazolinium Salts in Asymmetric Synthesis. 1. Stereoselective Reduc- tion Induced by a 3¢-Alkoxy Stereocentre. A New Approach to Poly- functionalizedb-Amino Acids. Z. Naturforsch. in press.
4. Jäger, V.; Le Roy, P.-Y.; Henneböhle, M.; Bathich, Y.; Remen, L.;
Imerhasan, M.:N-Methylisoxazolinium Salts – an Inconspicuous Class of Compounds with High Potential for Organic Synthesis. Presented at 6. Iminium-Salz-Tagung (ImSaT 6), p. 99-107. Stimpfach-Rechenberg, Germany, 16. to 18.09.2003.
194 (C
12H
22NO
4)Cl
5. Zhao, M.; Li, J.; Song, Z.; Desmond, R.; Tschaen, D. M.; Grabowski, E. J.
J.; Reider, P. J.: A Novel Chromium Trioxide Catalyzed Oxidation of Pri- mary Alcohols to the Carboxylic Acids. Tetrahedron Lett.39(1998) 5323-5326.
6. Sheldrick, G. M.: SHELXS-97. Program for the Solution of Crystal Structures. University of Göttingen, Germany 1997.
7. Sheldrick, G. M.: SHELXL-97. Program for the Refinement of Crystal Structures. University of Göttingen, Germany 1997.
8. Sheldrick, G. M.: SHELXTL-XP. Program for Molecular Graphics. Sie- mens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA 1990.