Crystal structure of methyl 3- O -acetyl-2-acetylamino-2,6-dideoxy-6-iodo- a - D -glucopyranoside, C 11 H 18 INO 6
Wolfgang Frey, Robert Sardzik and Volker Jäger
*Institut für Organische Chemie, Universität Stuttgart, Pfaffenwaldring 55, 70569 Stuttgart, Germany Received January 21, 2008, accepted and available on-line June 24, 2008; CCDC no. 1267/2275
Abstract
C
11H
18INO
6, monoclinic, P2
1(no. 4), a = 7.718(2) Å,
b = 9.419(3) Å, c = 10.755(2) Å, b = 104.73(2)°, V = 756.1 Å
3, Z = 2, R
gt(F) = 0.057, wR
ref(F
2) = 0.158, T = 293 K.
Source of material
The title compound has been obtained as a by-product of the reac- tion of methyl 3-O-acetyl-2-acetylamino- a -D-glucopyranoside with imidazole, triphenyl phosphane and iodine in toluene [1-4].
Isolation by column chromatography (dichloromethane/metha- nol) and crystallization from ethyl acetate/hexane afforded the ti- tle iodo compound as colourless crystals [1] (m.p. 431 - 433 K);
[ a ]
D20
= +76 (c = 0.53, CHCl
3).
Discussion
The absolute configuration, as known from the starting material, the natural product N-acetyl-D-glucosamine, was confirmed by the absolute structure determination by X-ray data indicated by the Flack parameter of x = − 0.03(3). The ring system of the pyranose moiety shows a chair conformation (figure, top). There is an intermolecular hydrogen bond between the hydroxy group O2 − H2A and the carbonyl oxygen O6 of the acetyl protecting
group. The H2A···O6 distance is 2.02 Å and the angle O2 − H2A···O6 is 155°. The polar layers in the (100) plane formed by the iodine atoms and the oxygen atoms of the pyranose moiety are stacked along [100]. In the same manner non-polar layers are evi- dent, built up by the carbon atoms of the molecules (figure, bot- tom).
Z. Kristallogr. NCS
223(2008) 255-256 /
DOI10.1524/ncrs.2008.0108
255©
by Oldenbourg Wissenschaftsverlag, München
Crystal: colourless block, size 0.7 × 0.8 × 1.2 mm Wavelength: MoKaradiation (0.71073 Å)
m: 21.38 cm−1
Diffractometer, scan mode: Nicolet P3, Wyckoff scan
2qmax: 65°
N(hkl)measured,N(hkl)unique: 5819, 5482 Criterion forIobs,N(hkl)gt: Iobs> 2s(Iobs), 4660 N(param)refined: 173
Programs: SHELXS-97 [5], SHELXL-97 [6],
SHELXTL [7]
Table 1.Data collection and handling.
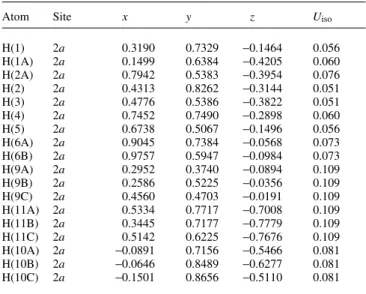
H(1) 2a 0.3190 0.7329 −0.1464 0.056
H(1A) 2a 0.1499 0.6384 −0.4205 0.060
H(2A) 2a 0.7942 0.5383 −0.3954 0.076
H(2) 2a 0.4313 0.8262 −0.3144 0.051
H(3) 2a 0.4776 0.5386 −0.3822 0.051
H(4) 2a 0.7452 0.7490 −0.2898 0.060
H(5) 2a 0.6738 0.5067 −0.1496 0.056
H(6A) 2a 0.9045 0.7384 −0.0568 0.073
H(6B) 2a 0.9757 0.5947 −0.0984 0.073
H(9A) 2a 0.2952 0.3740 −0.0894 0.109
H(9B) 2a 0.2586 0.5225 −0.0356 0.109
H(9C) 2a 0.4560 0.4703 −0.0191 0.109
H(11A) 2a 0.5334 0.7717 −0.7008 0.109
H(11B) 2a 0.3445 0.7177 −0.7779 0.109
H(11C) 2a 0.5142 0.6225 −0.7676 0.109
H(10A) 2a −0.0891 0.7156 −0.5466 0.081
H(10B) 2a −0.0646 0.8489 −0.6277 0.081
H(10C) 2a −0.1501 0.8656 −0.5110 0.081
Table 2.Atomic coordinates and displacement parameters (in Å2)
.
Atom Site x y z Uiso
_____________
* Correspondence author (e-mail: jager.ioc@oc.uni-stuttgart.de)
I(1) 2a 0.90827(5) 0.55245(5) 0.12698(3) 0.0788(2) 0.0882(3) 0.0460(2) 0.0158(2) −0.0077(1) 0.0008(2) O(1) 2a 0.5762(4) 0.6895(3) −0.1061(3) 0.049(1) 0.053(1) 0.042(1) 0.006(1) 0.005(1) −0.012(1) C(1) 2a 0.3994(5) 0.6753(4) −0.1832(4) 0.044(2) 0.047(2) 0.048(2) 0.008(1) 0.010(1) 0.001(1)
N(1) 2a 0.2074(4) 0.7235(3) −0.3990(4) 0.042(1) 0.033(1) 0.065(2) 0.000(1) 0.000(1) 0.010(1)
O(2) 2a 0.8278(5) 0.5579(7) −0.3249(3) 0.072(2) 0.125(3) 0.048(1) 0.052(3) 0.012(1) 0.000(2)
C(2) 2a 0.3896(5) 0.7276(3) −0.3196(4) 0.040(1) 0.036(1) 0.049(2) 0.001(1) 0.005(1) 0.005(1)
O(3) 2a 0.3394(4) 0.5337(3) −0.1938(3) 0.052(1) 0.053(2) 0.050(1) −0.006(1) 0.006(1) 0.010(1) C(3) 2a 0.5162(5) 0.6380(3) −0.3748(3) 0.047(2) 0.038(1) 0.039(1) 0.003(1) 0.005(1) −0.001(1) O(4) 2a 0.5219(4) 0.6935(3) −0.4993(3) 0.053(1) 0.045(1) 0.040(1) −0.004(1) 0.004(1) 0.0033(9) C(4) 2a 0.7036(5) 0.6508(5) −0.2893(4) 0.044(2) 0.061(2) 0.041(2) 0.010(1) 0.004(1) −0.004(1) O(5) 2a 0.3414(6) 0.5188(4) −0.6007(4) 0.075(2) 0.060(2) 0.055(2) −0.017(2) −0.000(2) −0.004(1) C(5) 2a 0.7024(5) 0.6079(4) −0.1520(4) 0.044(2) 0.051(2) 0.042(2) 0.007(1) 0.006(1) −0.005(1) C(6) 2a 0.8842(6) 0.6367(6) −0.0628(5) 0.046(2) 0.080(3) 0.048(2) −0.001(2) −0.001(2) −0.003(2) O(6) 2a 0.1862(6) 0.9579(3) −0.4342(4) 0.080(2) 0.038(1) 0.068(2) −0.002(1) −0.008(2) 0.005(1) C(7) 2a 0.1210(5) 0.8399(4) −0.4548(3) 0.047(2) 0.040(1) 0.039(1) 0.005(1) 0.009(1) 0.008(1)
C(8) 2a 0.4304(6) 0.6237(4) −0.6050(4) 0.049(2) 0.051(2) 0.043(2) 0.003(1) 0.001(1) 0.002(1)
C(9) 2a 0.3371(8) 0.4700(7) −0.0749(6) 0.066(3) 0.088(3) 0.066(3) −0.003(2) 0.020(2) 0.029(3) C(11) 2a 0.4580(9) 0.6897(8) −0.7232(5) 0.082(3) 0.086(3) 0.043(2) −0.010(3) 0.002(2) 0.008(2) C(10) 2a −0.0624(6) 0.8153(5) −0.5430(5) 0.048(2) 0.055(2) 0.054(2) 0.003(2) 0.002(2) 0.012(2) Table 3.Atomic coordinates and displacement parameters (in Å2).
Atom Site x y z U11 U22 U33 U12 U13 U23
Acknowledgments.We are grateful to Fonds der Chemischen Industrie and to Landesgraduierten-förderung Baden-Württemberg (Ph. D. scholarship to R.
Sardzik) for financial support.
References
1. Sardzik, R.: -Cyclopropanozucker- durch Vitamin B12-katalysierte Cyclisierung von Diiodiden; Carbonyldiimidazol als Reagenz für die Cyclisierung ungesättigter Aminoalkohole zu 3-Pyrrolinen. Dissertation, Universität Stuttgart (2007).
2. Garegg, P. J.; Samuelsson. B.: Novel Reagent System for converting a Hydroxy-group into an Iodo-group in Carbohydrates with Inversion of Configuration. J. Chem. Soc. Chem. Commun. (1979) 978-980.
3. Garegg, P. J.; Samuelsson. B.: Novel Reagent System for converting a Hydroxy-group into an Iodo-group in Carbohydrates with Inversion of Configuration, Part 2. J. Chem. Soc., Perkin Trans.1(1980) 2866-2869.
256
C
11H
18INO
64. Frey, W.; Sardzik, R.; Jäger, V.: Crystal structure of methyl 3-O-acetyl-2- [1-imidazol-1-yl-(E)-ethylideneamino]-2,4,6-trideoxy-4,6-diiodo-a-D- galactopyranoside, C14H19I2N3O4. Z. Kristallogr. NCS223(2008) 259- 261.
5. Sheldrick, G. M.: SHELXS-97. Program for the Solution of Crystal Struc- tures. University of Göttingen, Germany 1997.
6. Sheldrick, G. M.: SHELXL-97. Program for the Refinement of Crystal Structures. University of Göttingen, Germany 1997.
7. Sheldrick, G. M.: SHELXTL. Structure Determination Software Suite.
Version 6.14. Bruker AXS, Madison, Wisconsin, USA 2000.