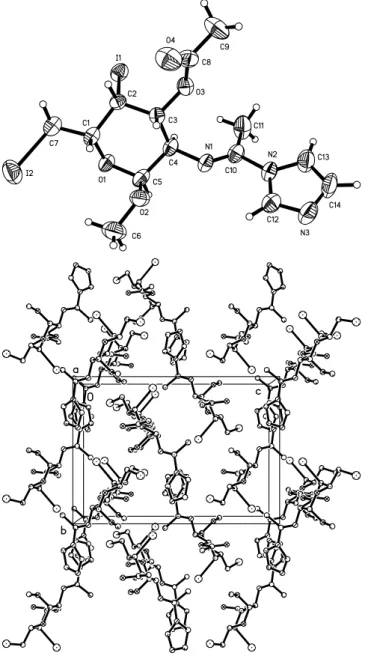
Crystal structure of methyl 3- O -acetyl-2-[1-imidazol-1-yl-( E )-
ethylideneamino]-2,4,6-trideoxy-4,6-diiodo- a - D -galactopyranoside, C 14 H 19 I 2 N 3 O 4
Wolfgang Frey, Robert Sardzik and Volker Jäger
*Universität Stuttgart, Institut für Organische Chemie, Pfaffenwaldring 55, 70569 Stuttgart, Germany Received January 21, 2008, accepted and available on-line June 24, 2008; CCDC no. 1267/2277
Abstract
C
14H
19I
2N
3O
4, orthorhombic, P2
12
12
1(no. 19), a = 10.445(2) Å, b = 11.327(2) Å, c = 15.937(3) Å, V = 1885.5 Å
3, Z = 4, R
gt(F) = 0.047, wR
ref(F
2) = 0.089, T = 293 K.
Source of material
The title compound has been obtained as a by-product by iodin- ation of methyl 3-O-acetyl-2-acetylamino- a -D-glucopyranoside with imidazole, triphenyl phosphine and iodine in toluene [1-4].
Isolation by column chromatography (dichloromethane/metha- nol) and crystallization from ethyl acetate/hexane gave the title diiodo-compound in the form of colourless crystals [1] (m.p. 434 - 437 K); [ a ]
D20= +119 (c = 0.780, CHCl
3).
Discussion
The absolute configuration a -D-galacto, as known from the natu- ral starting matrial, was confirmed by the absolute structure deter- mination by X-ray data indicated by the Flack parameter of x = − 0.03(3). This result confirms the steric course of the synthetic pathway. The N1=C10 double bond is clearly identified by the distance of 1.263(8) Å. The pyranose ring shows the expected chair conformation. The dihedral angle between the best plane of the pyranose moiety and the imidazole ring system is 63.6(2)°
(figure, top). The molecules are antiparallel oriented in [100], [010] and [001] forced by the perpendicular screw axis (figure, bottom).
Z. Kristallogr. NCS
223(2008) 257-258 /
DOI10.1524/ncrs.2008.0109
257©
by Oldenbourg Wissenschaftsverlag, München
Crystal: Colorless plates, size 0.01 × 0.1 × 0.2 mm Wavelength: MoKaradiation (0.71073 Å)
m: 33.57 cm−1
Diffractometer, scan mode: Nicolet P3, Wyckoff scan
2qmax: 59.98°
N(hkl)measured,N(hkl)unique: 6003, 5492 Criterion forIobs,N(hkl)gt: Iobs> 2s(Iobs), 4413 N(param)refined: 210
Programs: SHELXS-97 [5], SHELXL-97 [6],
SHELXTL [7]
Table 1.Data collection and handling.
H(1) 4a 0.1371 −0.1974 0.2742 0.044
H(2) 4a −0.0525 −0.2929 0.2499 0.039
H(3) 4a −0.0507 −0.1019 0.2109 0.040
H(4) 4a 0.0418 −0.1606 0.0478 0.044
H(5) 4a 0.2403 −0.0852 0.0870 0.049
H(6A) 4a 0.3073 0.0590 0.2703 0.105
H(6B) 4a 0.3682 0.0232 0.1841 0.105
H(6C) 4a 0.3418 −0.0734 0.2524 0.105
H(7A) 4a 0.2108 −0.4254 0.2234 0.056
H(7B) 4a 0.1333 −0.4040 0.3063 0.056
H(9A) 4a −0.3709 −0.1974 0.0694 0.104
Table 2.Atomic coordinates and displacement parameters (in Å2)
.
Atom Site x y z Uiso
_____________
* Correspondence author (e-mail: jager.ioc@oc.uni-stuttgart.de)
I(1) 4a −0.02250(4) −0.42147(3) 0.11320(3) 0.0582(3) 0.0313(2) 0.0463(2) −0.0017(2) −0.0016(2) −0.0026(2) O(1) 4a 0.2186(4) −0.2186(3) 0.1638(3) 0.041(2) 0.032(2) 0.045(2) 0.005(2) 0.008(2) 0.008(2) N(1) 4a 0.0053(5) 0.0082(4) 0.0752(3) 0.047(3) 0.028(2) 0.037(2) 0.003(2) −0.001(2) 0.003(2) C(1) 4a 0.1375(6) −0.2584(5) 0.2306(4) 0.045(3) 0.031(3) 0.034(3) 0.004(3) 0.006(3) 0.005(2) I(2) 4a 0.36968(5) −0.33513(5) 0.33394(4) 0.0557(3) 0.0694(3) 0.0776(4) 0.0133(3) −0.0206(3) 0.0011(3) N(2) 4a −0.0909(5) 0.1508(4) −0.0030(3) 0.050(3) 0.030(2) 0.036(2) 0.007(2) 0.001(2) 0.001(2) O(2) 4a 0.1868(4) −0.0198(4) 0.1942(3) 0.035(2) 0.038(2) 0.063(3) −0.003(2) −0.002(2) −0.003(2) C(2) 4a 0.0001(5) −0.2756(4) 0.2006(3) 0.035(3) 0.031(2) 0.031(2) −0.002(2) 0.003(2) −0.001(2) O(3) 4a −0.1690(4) −0.1685(4) 0.1257(2) 0.041(2) 0.040(2) 0.035(2) 0.003(2) −0.003(2) −0.001(2) C(3) 4a −0.0450(5) −0.1593(5) 0.1650(3) 0.033(3) 0.033(2) 0.033(2) 0.002(2) −0.001(2) 0.000(2) N(3) 4a −0.0950(6) 0.3438(5) 0.0188(4) 0.070(4) 0.034(3) 0.078(4) 0.007(3) 0.000(3) 0.007(3) O(4) 4a −0.2643(5) −0.1586(6) 0.2507(3) 0.056(3) 0.092(4) 0.049(3) −0.007(3) 0.008(2) −0.005(3) C(4) 4a 0.0447(6) −0.1099(4) 0.0976(3) 0.044(3) 0.026(2) 0.039(3) 0.005(2) 0.002(2) 0.003(2) C(5) 4a 0.1818(6) −0.1071(5) 0.1324(4) 0.043(3) 0.030(3) 0.050(4) 0.004(2) 0.013(3) 0.010(2) C(6) 4a 0.3111(7) −0.0012(7) 0.2280(6) 0.046(4) 0.058(4) 0.106(7) −0.009(4) −0.011(5) −0.004(5)
C(7) 4a 0.1946(7) −0.3689(5) 0.2679(4) 0.053(4) 0.041(3) 0.046(3) 0.002(3) 0.006(3) 0.007(3)
C(8) 4a −0.2712(6) −0.1712(6) 0.1766(4) 0.041(3) 0.039(3) 0.050(4) −0.006(3) 0.002(3) −0.008(3) C(9) 4a −0.3912(7) −0.1895(8) 0.1279(5) 0.040(4) 0.100(6) 0.068(5) −0.005(4) −0.009(3) −0.007(5) C(10) 4a −0.0586(6) 0.0295(5) 0.0097(4) 0.049(4) 0.033(3) 0.038(3) 0.005(3) 0.005(3) 0.001(2) C(11) 4a −0.1085(8) −0.0525(6) −0.0565(4) 0.083(6) 0.047(4) 0.054(4) 0.014(4) −0.021(4) −0.010(3) C(12) 4a −0.0419(7) 0.2439(5) 0.0405(4) 0.055(4) 0.036(3) 0.058(4) 0.000(3) −0.004(3) 0.000(3) C(13) 4a −0.1814(8) 0.1966(6) −0.0560(5) 0.073(5) 0.045(4) 0.054(4) 0.006(4) −0.020(4) 0.003(3) C(14) 4a −0.1830(8) 0.3132(6) −0.0417(5) 0.073(5) 0.045(4) 0.074(5) 0.016(4) −0.006(4) 0.022(4) Table 3.Atomic coordinates and displacement parameters (in Å2).
Atom Site x y z U11 U22 U33 U12 U13 U23
Acknowledgments.We are grateful to Landesgraduiertenförderung Baden- Württemberg (Ph. D. scholarship to R. Sardzik) and to Fonds der Chemischen Industrie for financial support.
References
1. Sardzik, R.: -Cyclopropanozucker- durch Vitamin B12-katalysierte Cyclisierung von Diiodiden; Carbonyldiimidazol als Reagenz für die Cyclisierung ungesättigter Aminoalkohole zu 3-Pyrrolinen. Dissertation, Universität Stuttgart (2007).
2. Garegg, P. J.; Samuelsson. B.: Novel Reagent System for converting a Hydroxy-group into an Iodo-group in Carbohydrates with Inversion of Configuration. J. Chem. Soc. Chem. Commun. (1979) 978-980.
3. Garegg, P. J.; Samuelsson. B.: Novel Reagent System for converting a Hydroxy-group into an Iodo-group in Carbohydrates with Inversion of Configuration, Part 2. J. Chem. Soc., Perkin Trans.1(1980) 2866-2869.
258
C
14H
19I
2N
3O
44. Frey, W.; Sardzik, R.; Jäger, V.: Crystal structure of methyl 3-O-acetyl-2- acetylamino-2,6-dideoxy-6-iodo-a-D-glucopyranoside, C11H18INO6. Z.
Kristallogr. NCS223(2008) 255/256.
5. Sheldrick, G. M.: SHELXS-97. Program for the Solution of Crystal Struc- tures. University of Göttingen, Germany 1997.
6. Sheldrick, G. M.: SHELXL-97. Program for the Refinement of Crystal Structures. University of Göttingen, Germany 1997.
7. Sheldrick, G. M.: SHELXTL. Structure Determination Software Suite.
Version 6.14. Bruker AXS, Madison, Wisconsin, USA 2000.
H(9B) 4a −0.4469 −0.1230 0.1357 0.104
H(9C) 4a −0.4331 −0.2599 0.1471 0.104
H(11A) 4a −0.0763 −0.1306 −0.0466 0.092
H(11B) 4a −0.0808 −0.0258 −0.1107 0.092
Table 2.Continued
.
Atom Site x y z Uiso
H(11C) 4a −0.2003 −0.0536 −0.0546 0.092
H(12) 4a 0.0219 0.2372 0.0809 0.060
H(13) 4a −0.2316 0.1552 −0.0942 0.069
H(14) 4a −0.2365 0.3664 −0.0690 0.077
Table 2.Continued
.
Atom Site x y z Uiso