32 Zeitschrift für Kristallographie - New Crystal Structures 213
© by R. Oldenbourg Verlag, München 1998
Crystal structure of dekagadolìiiium(III) oxide tetradekasulfide, GdioOSu
Th. Schleid and F. A. Weber
Universität Stuttgart, Institut für Anorganische Chemie, Pfaffenwaldring 55, D-70569 Stuttgart, Germany Received June 25, 1997, transferred to 1st update of database ICSD in 1998, CSD-No. 409047
Table 1. Parameters used for the X-ray data collection
Source of material: GdioOSn was obtained from С020з, gadoli- nium powder and sulfur (molar ratio 1:28:42) with equimolar amounts of CsCl or GdCb as fluxing agents (see refs. 1 and 2).
The mixtures were heated in sealed evacuated silica tubes at 973 К (7 d) and subsequently cooled down (50 K/d) to ambient tempera- ture. The refined X-ray powder diffraction data (a = 14.7437(6) Â, с = 19.571(1) Â) agree weU with those (a = 14.748(2) Â, с = 19.564(4) Â) previously reportedfor high-pressure prepared samples (see refs. 2 and 3).
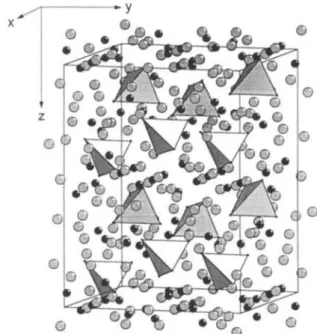
GdioOSi4 belongs to the PrioOSi4-type structure, see refs. 1 and 4-7. The crystal structure contains discrete [0(Gd(2))4]'°^tetra- hedra (d((>-Gd(2)) = 2.352(1)Â, 4 χ) embedded into a matrix ([GdeSul of the remainder gadolinium sulfide (see Fig., Gd( 1 ) and Gd(3): black, S: gray circles).
GdioOSR tetragonal, H\lacd (No. 142), a =14.747(2) Â, с =19572(3) À, V =4256.4 Â^ Ζ =8, R{F) =0.0Φ4, Äv/P) =0.092.
Table 2. Fmal atomic coordinates and displacement parameters (in h?)
Crystal: pale yellow, face-rich beads.
size 0.06 χ 0.07 χ 0.08 mm Wavelength: Mo Ka radiaüon (0.7107 Â)
μ: 3204 cm"'
Diffi^ctometer Siemens P3
Scan mode: profile fitted (iy20-scan
TmeasurfíwnJ'
293 К2Θ™„: 60°
ЩНкОилцие'·
1564 Criterion for Fa'- Fo>4a(Fo)bKparam)^„td·.
59Programs: SHELXS-86, SHELXL-93
Acknowledgments. We thank the Deutsche Forschungsgemeinschaft (Bonn) and the Fonds der Chemischen Industrie (Frankfurt/Main) for their financial support.
References
3.
5.
Schleid, Th.; Lissner, F.: MioSuO-type oxysulphides (M = La, Ce, Pr, Nd, Sm) as an "oxygen trap" in oxidation reactions of reduced lanthanide chlorides wiith sulphur. J. Less-Common Met. 175 (1991) 309-319.
Poxleitner, H.: Untersuchungen zur Polymorphie bei den Seltenerdses- quichalkogeniden LnaXs· Dissertation, Universität Regensburg, Germany
1988.
Range, K.-J.; Poxleitner, H.: Unpublished results.
4. Carré, D.; Laruelle, P.; Besançon, P.: Structure cristalline de la prétendue variété β des dulfures de terres rares de composition PrioSnO. Compt.
Rend. Acad. Sci. Paris 270 (1970) 537-539.
Besancon, P.: Teneur en Oxygène et Formule Exacte d'Une Famille de Composés Habituellement Appelés "Variété β " ou "Phase Complexe" des Sulftircs Terres Rares. J. SoUd State Chem. 7 (1973) 232-240.
6. Besançon, P.; Carré, D.; Laruelle, P.: Mécanisme de la Solution Solide des Oxysulfures de Terres Rares LioSis-xO,. Acta Crystallogr. B29 (1973) 1064-1066.
7. Bludau, W.; Wichelhaus, W.: Photoluminescence of doped LaioSwO. J.
Appi. Phys. 52 (1981) 2750-2755.
Sheldrick, G. M.: Phase Annealing in SHELX-90: Direct Methods for Large Structures. AcU Crystallogr. A46 (1990) 467-473.
Sheldrick, G. M.: SHELXL-93. Program for refining crystal structures.
University of Göttingen, Germany 1993.
8.
9.
Atom Site X
У ζ Un
ί/22 l/зз 1/12 {/13 t/23Gd(l) 32« 0.12761(3) 0.02762(4) 0.04689(3) 0.0241(3) 0.0202(3) 0.0185(3) 0.0008(2) 0.0006(2) 0.0004(2) Gd(2) 32« 0.36811(3) 0.25633(4) 0.05758(3) 0.0223(3) 0.0209(3) 0.0200(3) -0.0017(2) -0.0006(2) 0.0012(2)
Gd(3) 16/ 0.13513(3)
x+U4
1/8 0.0212(3)Uu
0.0186(3) 0.0005(2) -0.0001(2)-Ua
0 8o 0 1/4 3/8 0.019(4)
Uu
0.029(7) 0 0 0S(l) 32« 0.0249(2) 0.3820(2) 0.0038(1) 0.022(1) 0.026(1) 0.019(1) 0.0013(8) 0.0001(9) -0.0022(9) S(2) 32« 0.3440(2) 0.0729(2) 0.0931(1) 0.031(1) 0.020(1) 0.018(1) 0.0007(9) 0.0030(9) 0.0019(9) S(3) 32« 0.0383(2) 0.0706(2) 0.1718(1) 0.021(1) 0.022(1) 0.019(1) -0.0010(8) -0.0008(8) -0.0008(8)
S(4) 16e 0.3512(2) 0 1/4 0.023(1) 0.022(1) 0.021(1) 0 0 -0.003(1)