Z. Kristallogr. NCS 219 (2004) 287-288
© by Oldenbourg Wissenschaftsverlag, München
287
C r y s t a l s t r u c t u r e o f d i e t h y l r e / - ( 3 a S , 4 a S , 7 a / ? , 7 b / ? ) - 4 , 4 a , 7 a , 7 b - t e t r a h y d r o - 3 a / / - c y c l o p e n t a - [ l , 2 - J : 4 , 3 - i / ' ] d i i s o x a z o l e - 3 , 5 - d i c a r b o x y l a t e , C 1 3 H 1 6 N 2 O 6
W. Frey, J. Y. Lee and V. Jäger*
Universität Stuttgart, Institut fiir Organische Chemie, Pfaffenwaldring 55, 70569 Stuttgart, Germany Received March 30, 2004, accepted and available on-line July 28, 2004; CCDC no. 1267/1293
Abstract
C13H16N2O6, triclinic, P I (no. 2), a = 8.350(1) A,
b = 9.258(1) A, c = 10.933(2) A, a = 69.58(1)°,P = 79.79(1)°, y = 65.49(1)°, V= 720.0 A
3, Z = 2,Rgt(F) = 0.059, wRnfiF2) = 0.158, 7 = 293 K.
Source of material
The title compound was obtained by heating ethyl re/-(3a5,6aS)- 4,6a-dihydro-3a//-cyclopenta[<flisoxazole-3-carboxylate [1] with ethyl chlorooximidoacetate [2] in toluene under reflux [1,3]. The two diastereoisomers (ratio 52:48) were separated by column chromatography [4], whereby crystallization of the minor isomer from petroleum ether furnished the title compound in the form of colorless crystals (m.p. 372-373 K, dec.).
Discussion
The crystal structure confirms the anti arrangement of the rings.
Both isoxazoline moieties are nearly planar with a small degree of twist conformation. The central cyclopentane ring shows an en-
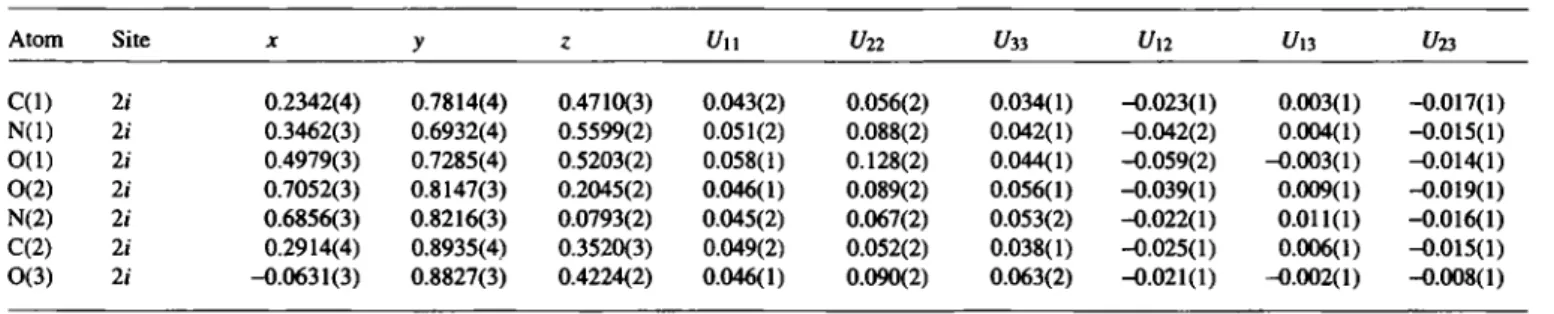
Table 3. Atomic coordinates and displacement parameters (in A2).
Atom Site x y z C(l) 2 i 0.2342(4) 0.7814(4) 0.4710(3) N(l) li 0.3462(3) 0.6932(4) 0.5599(2) O(l) 2i 0.4979(3) 0.7285(4) 0.5203(2) 0(2) 2 i 0.7052(3) 0.8147(3) 0.2045(2) N(2) li 0.6856(3) 0.8216(3) 0.0793(2) C(2) 2i 0.2914(4) 0.8935(4) 0.3520(3) 0(3) li -0.0631(3) 0.8827(3) 0.4224(2)
* Correspondence author (e-mail: jager.ioc@po.uni-stuttgart.de)
velope conformation. Both ethyl ester side-chains are planar with a slight out-of-plane orientation (9.5° and 14°) relative to the isoxazoline moieties to which they are connected.
Table 1. Data collection and handling.
Crystal: colorless block, size 0.2 x 0.2 x 0.5 mm Wavelength: Mo Ka radiation (0.71073 Â)
M- 1.09 c m- 1
Diffractometer, scan mode: Siemens-Nicolet P3, Wyckoff 50°
N(hkl¡measured, WlAOunique: 2717,2528 Criterion for /obs, N(hkl)p: /obs > 2 a(Iobs), 1668 N(param}rtfwd: 191
Programs: SHELXS-97 [5], SHELXL-97 [6]
Table 2. Atomic coordinates and displacement parameters (in Â2).
Atom Site X y z i/iso
H(2) 2i 0.2255 1.0113 0.3457 0.054
H(3) li 0.5160 0.9296 0.3737 0.064
H(5) li 0.4700 0.6149 0.2365 0.054
H(6) li 0.6697 0.6193 0.3481 0.061
H(7A) li 0.2767 0.9625 0.1502 0.056
H(7B) li 0.2000 0.8239 0.2248 0.056
H(9A) li -0.1620 0.6217 0.5427 0.077
H(9B) li -0.2200 0.7405 0.6299 0.077
H(10A) li -0.2296 0.4848 0.7532 0.133
H(10B) li -0.0873 0.5151 0.8060 0.133
H(10C) li -0.0294 0.3968 0.7193 0.133
H(12A) li 0.2714 0.8708 -0.2059 0.113
H(12B) li 0.3848 0.6811 -0.1892 0.113
H(13A) li 0.0941 0.7337 -0.1902 0.180
H(13B) li 0.0564 0.8008 -0.0705 0.180
H(13C) li 0.1695 0.6117 -0.0540 0.180
Un U22 Í/33 Un Un U23
0.043(2) 0.056(2) 0.034(1) -0.023(1) 0.003(1) -0.017(1) 0.051(2) 0.088(2) 0.042(1) -0.042(2) 0.004(1) -0.015(1) 0.058(1) 0.128(2) 0.044(1) -0.059(2) -0.003(1) -0.014(1) 0.046(1) 0.089(2) 0.056(1) -0.039(1) 0.009(1) -0.019(1) 0.045(2) 0.067(2) 0.053(2) -0.022(1) 0.011(1) -0.016(1) 0.049(2) 0.052(2) 0.038(1) -0.025(1) 0.006(1) -0.015(1) 0.046(1) 0.090(2) 0.063(2) -0.021(1) -0.002(1) -0.008(1)
288
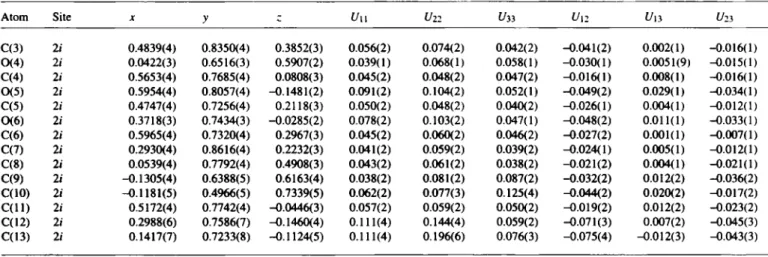
C l3H l 6 N20 6Table 3. Continued.
Atom Site X y Uu U22 Un Ur. Un Un
C(3) 2 i 0.4839(4) 0.8350(4) 0.3852(3) 0.056(2) 0.074(2) 0.042(2) -0.041(2) 0.002(1) -0.016(1) 0(4) 21 0.0422(3) 0.6516(3) 0.5907(2) 0.039(1) 0.068(1) 0.058(1) -0.030(1) 0.0051(9) -0.015(1) C(4) 21 0.5653(4) 0.7685(4) 0.0808(3) 0.045(2) 0.048(2) 0.047(2) -0.016(1) 0.008(1) -0.016(1) 0(5) 2 i 0.5954(4) 0.8057(4) -0.1481(2) 0.091(2) 0.104(2) 0.052(1) -0.049(2) 0.029(1) -0.034(1) C(5) 2 i 0.4747(4) 0.7256(4) 0.2118(3) 0.050(2) 0.048(2) 0.040(2) -0.026(1) 0.004(1) -0.012(1) CK6) 21 0.3718(3) 0.7434(3) -0.0285(2) 0.078(2) 0.103(2) 0.047(1) -0.048(2) 0.011(1) -0.033(1) C(6) 2 i 0.5965(4) 0.7320(4) 0.2967(3) 0.045(2) 0.060(2) 0.046(2) -0.027(2) 0.001(1) -0.007(1) C(7) 21 0.2930(4) 0.8616(4) 0.2232(3) 0.041(2) 0.059(2) 0.039(2) -0.024(1) 0.005(1) -0.012(1) C(8) 21 0.0539(4) 0.7792(4) 0.4908(3) 0.043(2) 0.061(2) 0.038(2) -0.021(2) 0.004(1) -0.021(1) C(9) 2 i -0.1305(4) 0.6388(5) 0.6163(4) 0.038(2) 0.081(2) 0.087(2) -0.032(2) 0.012(2) -0.036(2) C(10) 2i -0.1181(5) 0.4966(5) 0.7339(5) 0.062(2) 0.077(3) 0.125(4) -0.044(2) 0.020(2) -0.017(2) C ( l l ) 2 i 0.5172(4) 0.7742(4) -0.0446(3) 0.057(2) 0.059(2) 0.050(2) -0.019(2) 0.012(2) -0.023(2) C(12) 2i 0.2988(6) 0.7586(7) -0.1460(4) 0.111(4) 0.144(4) 0.059(2) -0.071(3) 0.007(2) -0.045(3) C(13) 2/ 0.1417(7) 0.7233(8) -0.1124(5) 0.111(4) 0.196(6) 0.076(3) -0.075(4) -0.012(3) -0.043(3)
Acknowledgments. We are grateful to Fonds der Chemischen Industrie and to Bayer AG for financial support.
References
1. Lee, J.-Y.: Synthese neuartiger Furanomycin-Analoga und Versuche zur Synthese von Diaminodicarbonsäuren über Bis(isoxazoline). Disserta- tion. Universität Stuttgart, Germany, in preparation.
2. Skinner, G. S.: Deaminization. HI. Evidence of the Existence of Aliphatic Diazonium Salts From the Formation of Chlorooximino Compounds.
J. Am. Chem. Soc. 46 (1924) 731-741.
3. de Micheli, C.; Caldirola, P.; de Amici, M.: An Efficient Synthesis of Isoxazoles and Isoxazolines. Heterocycles 23 (1985) 2479-2482.
4. Frey, W.; Lee, J.-Y.; Jäger, V.: Crystal Structure of diethyl rel-(3aR, 3bÄ,6a5,7aÄ)-3b,6a,7,7a-tetrahydro-3aff-cyclopenta[l,2-<i:3,4-i/']di- isoxazole-3,6-dicarboxylate, C13H16N2O6. Z. Kristallogr. NCS 213 (2004) 289-290.
5. Sheldrick, G. M.: SHELXS-97. Program for the Solution of Crystal Structures. University of Göttingen, Germany 1997.
6. Sheldrick, G. M.: SHELXL-97. Program for the Refinement of Crystal Structures. University of Göttingen, Germany 1997.