ζ . Kristallogr. NCS 213 (1998) 577-578
577
© by R. Oldenbourg Verlag, München
C r y s t a l s t r u c t u r e o f ( Z ) - 2 , 3 - 0 - i s o p r o p y l i d e n e - D - ^ 7 3 ' i / i r o - 4 - p e n t e n o s e · o x i m e , C s H o N O s
W. Frey, L. Bierer, D. Shaw and V. Jäger
Universität Stuttgart. Institut für Organische Chemie, Pfaffenwaldring 55, D-70569 Stuttgart, Germany Received Febraary 20, 1998, CSD-No. 409241
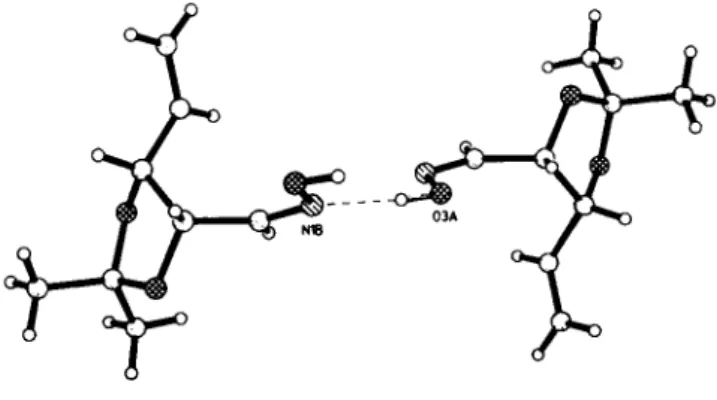
Flg. 1. Molecule plot.
Source of material: The title compound was prepared by oxim- ation of the corresponding aldehyde (see ref. 1-3) and was separ- ated from the E-oxime by MPLC. Crystallization from petroleum ether/ethyl acetate (4:1) furnished the title compound in the form of colorless crystals [mp 362 К - 363 К, [α]^ = +216 (с = 0.545, CH2CI2)].
The dioxolane rings of the molecules are stacked face-to-face in two antiparallel columns connected by intermolecular ( 0 3 A - H3A - N1B) hydrogen bonds between the oxime groups. The hydrogen bond bridges form a zig-zag chain between the mole- cules in the crystal.
C8H13NO3, orthorhombic, P2i2i2i (No. 19) a =5.229(1) Л,
b =12.281(2) Â, с =14.878(2) Â, V =955.4 Â^ Ζ =4, R(F) =0.052,ÄwCF^) =0.134.
Fig. 2. Hydrogen bonds between the oxime groups.
Table 1. Parameters used for the X-ray data collection
Ciystal: colorless block, size 0.3 χ 0.6 χ 1.7 mm Wavelength: Mo Ka radiation (0.71073 Â)
μ: 0.91 cm"'
Difiractometer: Nicolet P3
Scan mode: Wyckoff
Tifi^asuK/Miu· 293 К 65°
NChkOu^u.: 1887
Criterion for Jo: /ο>2σ(/ο) N(param),φκ<Γ• 110
Programs: SHELXS-86, SHELXL-93
Table 2. Final atomic coordinates and displacement parameters (in Л^)
Atom Site X У ζ
í /iso
H(1A) 4u 1.2129(9) -0.1827(2) -0.0832(2) 0.101 H(1B) 4ϋ 1.4201(9) -0.1181(2) -0.1430(2) 0.101 H(2) 4a 1.5629(6) -0.0341(2) -0.0215(2) 0.073
H(3A) 4a 0.7954 0.1988 -0.0006 0.065
H(3) 4a 1.2158(5) -0.1693(1) 0.0755(1) 0.060 H(4) 4a 0.9837(4) -0.0172(1) 0.1066(1) 0.051 H(5) 4a 1.4270(4) 0.1181(1) 0.0996(1) 0.053 H(7A) 4a 1.437(1) -0.2495(3) 0.2749(2) 0.163 H(7B) 4a 1.179(1) -0.2257(3) 0.2247(2) 0.163 H(7C) 4a 1.225(1) -0.1795(3) 0.3217(2) 0.163 H(8A) 4a 1.7572(6) -0.0941(4) 0.2946(2) 0.150 H(8B) 4a 1.5476(6) -0.0218(4) 0.3407(2) 0.150 H(8C) 4a 1.6893(6) 0.0204(4) 0.2546(2) 0.150
578
(Z)-2,3-0-Isopropylidene-D-erythro-4-pentenose-oximeТаЫе 3. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X У ζ f/ll (/22 Un Ur. Un Uli
N(l) 4a 1.1328(3) 0.1733(1) 0.0443(1) 0.0444(8) 0.0318(5) 0.0507(8) -0.0041(6) 0.0040(7) 0.0036(5) 0(1) 4a 1.5247(3) -0.1148(1) 0.1389(1) 0.0622(9) 0.0640(9) 0.0505(7) 0.0217(8) 0.0010(7) 0.0077(6) C(l) 4a 1.3462(9) -0.1328(2) -0.0874(2) 0.121(3) 0.076(2) 0.057(1) -0.005(2) -0.003(2) -0.004(1) 0(2) 4a 1.2217(3) -0.0165(1) 0.21172(9) 0.0571(8) 0.0674(9) 0.0441(6) 0.0163(8) 0.0057(7) O.Ol 19(6) C(2) 4a 1.4295(6) -0.0836(2) -0.0156(2) 0.083(2) 0.0467(9) 0.052(1) 0.001(1) 0.002(1) 0.0015(8) 0(3) 4a 0.8833(3) 0.1414(1) 0.0245(1) 0.0472(7) 0.0384(6) 0.076( 1 ) -0.0018(6) -0.0101(7) 0.0184(6) C(3) 4a 1.3204(5) -0.1030(1) 0.0760( 1 ) 0.065(1) 0.0356(7) 0.0503(9) -0.0019(8) -0.0032(9) 0.0061(7) C(4) 4a 1.1671(4) -0.0084(1) 0.1179(1) 0.0398(7) 0.0391(7) 0.0485(8) -0.0018(6) -0.0015(7) 0.0147(7) C(5) 4a 1.2578(4) 0.1011(1) 0.0865( 1 ) 0.0402(8) 0.0370(7) 0.0544(9) -0.0052(7) -0.0004(7) 0.0059(7) C(6) 4a 1.4190(5) -0.0947(2) 0?757(l) 0.052(1) 0.074(1) 0.0459(9) 0.018(1) 0.0014(8) 0.0169(9) C(7) 4a 1.305(1) -0.1966(3) 0.2653(2) 0.156(4) 0.079(2) 0.091(2) 0.009(2) 0.015(3) 0.049(2) C(8) 4a 1.6218(6) -0.0428(4) 0.2842(2) 0.058(2) 0.182(4) 0.060(1) 0.012(2) -0.007(1) -0.017(2)
Acknowledgments. We are grateful to A. v. Humboldt-Stiftung, Bonn for awarding a post-doctoral fellowship to D. Shaw (1994/95), to Volkswagen- Stifhing, Hannover, and to Fonds der Chemischen Industrie for financial support.
References
1. Jäger, v.; Shaw, D.: Unpublished lesults, 1995.
2. Bierer, L.: Dissertation, Universität Stuttgart. In preparation.
3. Bierer, L.; Jäger, V.; Shaw, D.: Manuscript in preparation.
4. cf Gallos. J. K.; Goga, E. G.; Koumbis, A. E.: Expeditious Synthesis of Aminocyclopentitols from D-Ribose via Intramolecular Nitrone Cycload- dition. J. Chem. Soc. Perkin Trans. I (1994) 613-614.
Paquette, L. Α.; Bailey, S.: Evaluation of D-Ribose as an Enantiopure Building Block for Construction of the C-Ring of Taxol and Its Congeners.
J. Org. Chem. 60 (1995) 7849-7856.
Sheldrick, G. M.: Phase Annealing in SHELX-90: Direct Methods for Large Structures. Acta Crystallogr. A46 ( 1990) 467-473.
Sheldrick, G. M.: SHELXL-93. Program for refming crystal structures.
University of Göttingen, Germany 1993.
5.
6.
7.