Ζ . Kristallogr. N C S 2 1 5 ( 2 0 0 0 ) 1 2 7 - 1 2 8
© b y O l d e n b o u r g W i s s e n s c h a f t s v e r l a g , M ü n c h e n
1 2 7
Crystal structure of (25,3R,4R,55,17?,2'5)-3,4-dimethanesulfonyloxy- 2- [2,3-0-isopropylidenedioxy-1 -(teri-butoxycarbonylamino)propyl] - 5-methyl-tetrahydrofuran ethyl acetate, C18H33NO11S2 · C 4 H 8 02
W. Frey, P. J. Zimmermann and V. Jäger*
Universität Stuttgart, Institut für Organische Chemie, Pfaffenwaldring 55, D-70569 Stuttgart, Germany Received July 14, 1999, CCDC-No. 1267/224
Table 1. Data collection and handling.
Abstract
C22H41NO13S2, m o n o c l i n i c , P 1 2 i l ( N o . 4 ) , α = 1 2 . 4 0 7 ( 2 ) Ä , b = 9 . 2 5 4 ( 1 ) Ä , с = 1 3 . 8 1 2 ( 1 ) Ä , β = 1 0 4 . 8 4 ( 1 ) ° , V= 1 5 3 3 . 0 Ä3, Ζ = 2, Rgi(F) = 0 . 0 6 6 , WRVJ(F2) = 0 . 1 6 0 , Τ = 2 9 3 К .
Source of material
T h e t i t l e c o m p o u n d w a s p r e p a r e d b y r e d u c t i o n o f t h e c o r r e s - p o n d i n g i s o x a z o l i n e [ 1 - 7 ] u s i n g L i A l t U . A f t e r p r o t e c t i o n o f t h e a m i n e a s c a r b a m a t e , t h e r e s u l t i n g /V-Вос p r o t e c t e d a m i n o d i o l w a s d i m e s y l a t e d . C r y s t a l l i z a t i o n f r o m p e t r o l e u m e t h e r / e t h y l a c e t a t e ( 4 : 1 ) f u r n i s h e d t h e t i t l e c o m p o u n d a s a n e t h y l a c e t a t e s o l v a t e i n t h e f o r m o f c o l o u r l e s s c r y s t a l s ( m p 3 6 1 К - 3 6 2 Κ , [ α ] ^ = - 4 . 2 , с = 1 . 5 0 , C H C b ) .
Discussion
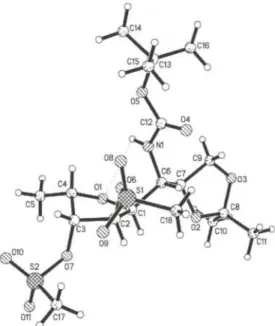
T h e c o m p o u n d c r y s t a l l i z e s w i t h o n e d i s o r d e r e d e t h y l a c e t a t e m o l e c u l e i n t h e a s y m m e t r i c u n i t . N 1 — O l f o r m s a w e a k i n t r a m o l e c u l a r h y d r o g e n b o n d w i t h a H l — O l d i s t a n c e o f 2 . 5 8 Ä a n d a n N l - H l - O l a n g l e o f 9 5 . 7 ° , g e n e r a t i n g a five-membered ring s y s t e m ( Ν 1 , H 1 , 0 1 , C 1 , C 6 ) w i t h e n v e l o p e c o n f o r m a t i o n . W e a l s o o b s e r v e a n i n t e r m o l e c u l a r h y d r o g e n b o n d w i t h N 1 — H I a s d o n o r a n d О З o f t h e d i o x o l a n e g r o u p a s a c c e p t o r . T h e H I — О З d i s t a n c e is 2 . 2 4 Ä , t h e N 1 - H 1 - 0 3 a n g l e is 1 6 0 . 8 ° . T h e p o l a r s u l f o n y l o x y m o i e t i e s a r e o r i e n t e d t o w a r d s t h e s o l v e n t m o l e c u l e s .
Crystal: colourless plates, size 0.15 χ 0.3 χ 1.2mm
Wavelength: Mo Ka radiation (0.71073 Ä)
μ: 2.33 cm"1
Diffractometer, scan mode: Nicolet P3, Wyckoff
20max: 55°
N(hkl)measured, N(hkl)mique: 3904, 3737 Criterion for /0bs, N(hkl)gl: U s > 2 af/obs), 2911
N(paramhefmeA'. 348
Programs: SHELXS-86 [8], SHELXL-93 [9]
Table 2. Atomic coordinates and displacement parameters (in Ä2).
Atom Site Occ. χ H ( l )
H(1A) H(2) H(3) H(4) H(5A) H(5B) H(5C) H(6) H(7) H(9A) H(9B) H(10A H(10B H(10C H(11A H(11B H(11C H(14A H(14B H(14C H(15A H(15B H(15C H(16A H(16B H(16C H(17A H(17B H(17C H(18A H(18B H(18C H(1L1 H(1L2 H(2L1 H(2L2
2 a 2 a 2 a 2a 2a 2 a 2 a 2a 2a 2a 2a 2 a 2 a 2 a 2 a 2a 2a 2a 2 a 2 a 2a 2a 2a 2a 2a 2 a 2 a 2 a 2a 2a 2a 2a 2 a 2a 2a 2a 2a
0.8242(3) 1.0053(3) 0.8738(4) 0.7557(4) 0.7799(4) 0.8834(6) 0.8147(6) 0.9422(6) 0.8919(3) 1.0411(4) 0.9808(4) 0.9504(4) 1.3170(6) 1.3178(6) 1.2481(6) 1.2456(7) 1.1364(7) 1.2470(7) 0.4795(6) 0.5484(6) 0.4318(6) 0.4668(6) 0.4159(6) 0.5231(6) 0.632(1) 0.628(1) 0.521(1) 0.7936(7) 0.9221(7) 0.8571(7) 0.7994(8) 0.6804(8) 0.7301(8) 0.7382 0.7890 0.5023 0.5517
0.8135(5) 0.8204(6) 0.8279(6) 0.5990(6) 0.6016(7) 0.4025(8) 0.3606(8) 0.3956(8) 1.0129(6) 0.8723(7) 1.0477(8) 1.1526(8) 0.984(1
1.074(1 0.931(1 1.197(1 1.274(1 1.293(1 0.796(1 0.851(1 0.920(1 0.929(2 1.066(2 1.081(2 1.098(2 1.187(2 1.173(2 0.653(1 0.674(1 0.558(1 1.087(1 1.153(1 1.139(1 0.2365 0.2701 0.2854 0.3165
0.0269(3) 0.2714(3) 0.3538(3) 0.2976(3) 0.1372(4) 0.1132(5) 0.1899(5) 0.2280(5) 0.1791(3) 0.0833(3) -0.0301(4) 0.0491(4) 0.2079(7) 0.1117(7) 0.1025(7) 0.2868(6) 0.2277(6) 0.1939(6) -0.1419(6) -0.2152(6) -0.2187(6) -0.0007(7) -0.0627(7) 0.0261(7) -0.1885(9) -0.0930(9) -0.1819(9) 0.5074(5) 0.5523(5) 0.5983(5) 0.3688(6) 0.3564(6) 0.2633(6) 0.6852 0.5949 0.5501 0.4582
0.047 0.041 0.047 0.048 0.054 0.108 0.108 0.108 0.043 0.049 0.060 0.060 0.153 0.153 0.153 0.152 0.152 0.152 0.166 0.166 0.166 0.192 0.192 0.192 0.219 0.219 0.219 0.122 0.122 0.122 0.138 0.138 0.138 0.170 0.170 0.249 0.249 Correspondence author (e-mail: jager.ioc@po.uni-stuttgart.de)
128
C 1 8 H 3 3 N O 1 1 S 2 C 4 H 8 O 2T a b l e 2. Continued.
A t o m Site Осе. χ у ζ f i s o
H ( 3 L I ) 2 а 0.66 0.873(1) 0.088(2) 0.684(1) 0.257 H ( 3 L 2 ) 2 а 0.66 0.814(1) 0.039(2) 0.575(1) 0.257 H ( 3 L 3 ) 2 а 0.34 0.761(1) 0.001(2) 0.663(1) 0.257
Table 2. Continued.
Atom Site Occ. χ у ζ U\sa
H(4L1) 2 a 0 . 3 4 0.569(1) 0.514(1) 0.5609(8) 0.369 H(4L2) 2 a 0.686(1) 0.457(1) 0.5541(8) 0.369 H(4L3) 2 a 0.644(1) 0.426(1) 0.6500(8) 0.369
T a b l e 3 . Atomic coordinates and displacement parameters (in Ä2).
A t o m Site χ у ζ U\\ U22 Чъъ U\2 Vn U2 3
S ( l ) 2
a
0.6672(1) 0.9239(3) 0.3008(1) 0.0579(8) 0.0572(8) 0.0740(9) 0.0084(7) 0.0371(7) - 0 . 0 0 0 1 ( 8 ) 0 ( 1 ) 2a
0.9362(3) 0.6660(5) 0.1775(2) 0.051(2) 0.041(2) 0.039(2) 0.005(2) 0.019(1) - 0 . 0 0 0 ( 1 ) N ( l )la
0.8209(3) 0.8911(5) 0.0604(3) 0.040(2) 0.040(2) 0.036(2) 0.002(2) 0.005(2) - 0 . 0 0 6 ( 2 ) C ( ! )2 a
0.9324(3) 0.8040(6) 0.2245(3) 0.031(2) 0.039(2) 0.031(2) - 0 . 0 0 3 ( 2 ) 0.005(2) - 0 . 0 0 2 ( 2 )S(2)
2a
0.8887(2) 0.4764(4) 0.4556(1) 0.118(2) 0.0551(8) 0.0489(7) 0.018(1) 0.0307(8) 0.0163(7)0 ( 2 )
2 a
1.1010(3) 1.0093(6) 0.1943(2) 0.047(2) 0.086(3) 0.042(2) - 0 . 0 2 2 ( 2 ) 0.006(2) 0.016(2) C(2)2a
0.8460(4) 0.7876(6) 0.2864(3) 0.040(2) 0.045(3) 0.035(2) 0.001(2) 0.014(2) - 0 . 0 0 1 ( 2 ) 0 ( 3 )2 a
1.1108(4) 1.1426(7) 0.0584(3) 0.073(3) 0.114(4) 0.057(2) - 0 . 0 4 3 ( 3 ) 0.002(2) 0.037(3) C(3) 2a
0.8296(4) 0.6251(6) 0.2898(3) 0.037(2) 0.045(3) 0.037(2) - 0 . 0 0 1 ( 2 ) 0.010(2) 0.003(2) C(4)2a
0.8469(4) 0.5774(7) 0.1898(4) 0.048(3) 0.043(3) 0.045(2) - 0 . 0 0 3 ( 2 ) 0.009(2) - 0 . 0 0 4 ( 2 ) 0 ( 4 )2 a
0.7196(3) 1.0922(6) 0.0688(3) 0.064(2) 0.052(2) 0.071(2) 0.018(2) 0.004(2) - 0 . 0 1 0 ( 2 ) 0 ( 5 )2a
0.6571(3) 0.9151(6) - 0 . 0 4 5 8 ( 3 ) 0.043(2) 0.061(2) 0.064(2) 0.011(2) - 0 . 0 0 5 ( 2 ) - 0 . 0 0 6 ( 2 ) C(5)2a
0.8743(6) 0.4196(8) 0.1793(5) 0.103(5) 0.046(3) 0.075(4) - 0 . 0 0 1 ( 4 ) 0.037(4) - 0 . 0 1 3 ( 3 )0 ( 6 ) 2
a
0.7407(3) 0.8523(5) 0.2345(3) 0.043(2) 0.053(2) 0.049(2) 0.007(2) 0.018(2) 0.000(2)C(6)
2a
0.9123(3) 0.9257(6) 0.1476(3) 0.035(2) 0.040(2) 0.034(2) - 0 . 0 0 1 ( 2 ) 0.008(2) 0.003(2) C(7)2 a
1.0160(4) 0.9594(7) 0.1115(3) 0.035(2) 0.050(3) 0.039(2) - 0 . 0 0 4 ( 2 ) 0.011(2) 0.000(2)0 ( 7 )
2 a
0.9187(3) 0.5695(6) 0.3697(3) 0.056(2) 0.072(3) 0.043(2) 0.012(2) 0.016(2) 0.017(2)0 ( 8 )
2 a
0.5584(4) 0.9360(8) 0.2360(4) 0.047(2) 0.117(5) 0.122(4) 0.012(3) 0.028(2) - 0 . 0 1 4 ( 4 ) C(8)2 a
1.1738(4) 1.0998(8) 0.1553(4) 0.051(3) 0.078(4) 0.049(3) - 0 . 0 2 2 ( 3 ) 0.014(2) 0.014(3) C(9)2 a
1.0042(4) 1.0824(8) 0.0383(4) 0.046(3) 0.064(3) 0.042(2) - 0 . 0 0 3 ( 3 ) 0.013(2) 0.008(3)0 ( 9 )
2a
0.6848(5) 0.8441(8) 0.3917(4) 0.123(4) 0.102(4) 0.094(3) 0.033(4) 0.078(3) 0.027(3)O ( 1 0 ) 2
a
0.7902(7) 0.3969(8) 0.4144(4) 0.221(8) 0.085(4) 0.078(3) - 0 . 0 8 1 ( 5 ) 0.055(4) - 0 . 0 0 2 ( 3 ) C(10)2a
1.2732(6) 1.015(1) 0.1433(7) 0.066(4) 0.125(8) 0.126(7) - 0 . 0 0 2 ( 5 ) 0.046(5) 0.021(6)O d 1)
2 a
0.9899(9) 0.407(1) 0.5009(5) 0.203(7) 0.165(7) 0.086(3) 0.114(6) 0.060(4) 0.068(4)C ( l l )
2 a
1.2033(7) 1.227(1) 0.2218(6) 0.098(6) 0.119(7) 0.094(5) - 0 . 0 6 1 ( 6 ) 0.039(5) - 0 . 0 1 9 ( 5 ) C ( 1 2 )2a
0.7317(4) 0.9783(7) 0.0316(3) 0.045(3) 0.041(2) 0.041(2) - 0 . 0 0 1 ( 2 ) 0.007(2) 0.005(2) C ( 1 3 )2a
0.5552(5) 0.9928(9) - 0 . 0 9 8 2 ( 5 ) 0.049(3) 0.084(5) 0.067(3) 0.023(3) - 0 . 0 0 5 ( 3 ) 0.008(4) C ( 1 4 ) 2a
0.4984(6) 0.879(1) - 0 . 1 7 5 8 ( 6 ) 0.065(4) 0.143(9) 0.094(5) 0.018(5) - 0 . 0 3 4 ( 4 ) - 0 . 0 2 8 ( 6 ) C(15)2 a
0.4839(6) 1.020(2) - 0 . 0 2 7 5 ( 7 ) 0.049(4) 0.21(1) 0.116(7) 0.038(6) 0.008(4) - 0 . 0 4 6 ( 8 ) C O 6)2 a
0.587(1) 1.124(2) - 0 . 1 4 4 4 ( 9 ) 0.146(9) 0.13(1) 0.136(9) 0.024(8) - 0 . 0 0 8 ( 7 ) 0.074(8) C O 7)2 a
0.8625(7) 0.605(1) 0.5375(5) 0.118(6) 0.078(5) 0.053(3) - 0 . 0 0 9 ( 4 ) 0.030(4) - 0 . 0 0 7 ( 3 ) C O 8)2 a
0.7258(8) 1.095(1) 0.3251(6) 0.120(6) 0.066(5) 0.100(6) 0.004(5) 0.046(5) - 0 . 0 2 3 ( 4 ) O d L)2 a
0.6433(6) 0.190(1) 0.5575(6) 0.093(5) 0.24(1) 0.132(6) 0.051(7) 0.014(4) - 0 . 0 1 9 ( 7 )C( 1L)
2 a
0.743(1) 0.199(1) 0.622(1) 0.111(9) 0.19(2) 0.120(9) - 0 . 0 1 ( 1 ) 0.021(7) 0.03(1)C ( 2 L )
2 a
0.570(2) 0.303(3) 0.534(2) 0.22(1) 0.21(1) 0.20(1) 0.032(9) 0.053(9) - 0 . 0 2 8 ( 9 ) C ( 3 L )2a
0.802(1) 0.072(2) 0.637(1) 0.098(8) 0.16(1) 0.22(1) 0.022(9) - 0 . 0 3 7 ( 8 ) - 0 . 0 1 ( 1 )C ( 4 L )
2 a
0.621(1) 0.436(1) 0.5784(8) 0.29(2) 0.26(2) 0.21(1) - 0 . 0 1 ( 2 ) 0.11(1) 0.06(1)0 ( 5 L )
2 a
0.489(1) 0.298(1) 0.4538(8) 0.16(1) 0.31(2) 0.14(1) 0.07(1) 0.025(9) 0.00(1)0 ( 6 L )
2 a
0.758(1) 0.327(1) 0.6356(8) 0.20(3) 0.09(2) 0.09(1) - 0 . 0 6 ( 2 ) 0.04(1) - 0 . 0 3 ( 1 )A c k n o w l e d g m e n t s . W e are g r a t e f u l to the L a n d e s g r a d u i e r t e n f ö r d e r u n g B a d e n - W ü r t t e m b e r g (doctoral fellowship to P. J. Z i m m e r m a n n ) . For finan- cial support of this work w e thank Volkswagenstiftung (Hannover), Fonds der C h e m i s c h e n Industrie, and Bayer A G , Wuppertal.
References
1. Jäger, V.; Müller, I.: Synthesis of A m i n o S u g a r s via Isoxazolines. Nitrile O x i d e - Furan Adducts as Key Intermediates in the Isoxazoline Route T o - w a r d s N o v e l A m i n o S u g a r D e r i v a t i v e s . T e t r a h e d r o n 4 1 ( 1 9 8 5 ) 3 5 1 9 - 3 5 2 8 .
2. Z i m m e r m a n n , P. J.: Furo[2,3-d]isoxazoline - Schlüsselbausteine in der Synthese von L-(+)-Furanomycin und D e s o x y n o j i r i m y c i n - H o m o l o g e n . Dissertation, Universität Stuttgart, in preparation.
3. Z i m m e r m a n n , P. J.; Blanarikova, I.; Jäger, V.: A General Approach to L-(+)-Furanomycin, S o m e Stereoisomers, and A n a l o g u e s Using Furo- isoxazoline Intermediates. A n g e w . Chem., in print.
4. Jäger, V.; Leibold, Т.; M ü l l e r , R.; Svete, J.; Z i m m e r m a n n , P. J.:
F u r o [ 2 , 3 - d ] i s o x a z o l i n e s - K e y I n t e r m e d i a t e s in the S y n t h e s i s of Biologically Active Compounds. Book of abstracts, 17th ICHC Vienna, 1999.
5. Müller, I.; Jäger, V.: Synthesis of A m i n o Sugars via Isoxazolines. T h e C o n c e p t and O n e Application: Nitrile Oxide/Furan Adducts. Tetrahedron Lett. 2 3 (1982) 4777-4780.
6. Jäger, v . ; Grund, Η.; Büß, V.; Schwab, W.; Müller, I.; Schohe, R.; Franz, R.; Ehrler, R.: Isoxazolines - Key Intermediates f o r Syntheses of S o m e Naturally Occuring A m i n o C o m p o u n d s . Bull. Soc. Chim. Belg. 9 2 (1983)
1039-1054.
7. Müller, R.; Leibold, Т.; Pätzel, Μ.; Jäger, V.: Eine neue Synthese von 1,3,4-Tridesoxy-1,4-iminoglyciten mit variabler Kettenlänge durch (C3 + Cn) - V e r k n ü p f u n g v o n A l l y l h a l o g e n i d e n und G l y c o n o n i t r i l o x i d e n . A n g e w . Chem. 106 (1994) 1305-1308; A n g e w . C h e m . Int. Ed. Engl. 3 3 (1994) 1295-1298.
8. Sheldrick, G. M . : Phase Annealing in S H E L X - 9 0 : Direct Methods for Larger Structures. Acta Crystallogr. A 4 6 (1990) 467-473.
9. Sheldrick, G. M . : S H E L X L - 9 3 . Program f o r the R e f i n e m e n t of Crystal Structures. University of Göttingen, G e r m a n y 1993.