Ζ. Kristallogr. N C S 2 1 5 ( 2 0 0 0 ) 1 2 9 - 1 3 0
© b y O l d e n b o u r g W i s s e n s c h a f t s v e r l a g , M ü n c h e n
129
Crystal structure of (2S,3/?,4S,17?,2'S)-3,4-dimethanesulfonyloxy- 2-f2,3-0-isopropylidenedioxy-l-(^ri-butoxycarbonylamino)propyl]- 5,5-dimethyl-tetrahydrofuran, C19H35NO11S2
W. Frey, I. Blanarikova, P. J. Zimmermann and V. Jäger*
Universität Stuttgart, Institut für Organische Chemie, Pfaffenwaldring 55, D-70569 Stuttgart, Germany Received July 14, 1999, C C D C - N o . 1267/225
Abstract
C19H35NO11S2, t r i c l i n i c , PI ( N o . 1), a = 8 . 2 0 1 ( 2 ) Ä ,
b = 9 . 3 6 8 ( 2 ) Ä , с = 1 0 . 1 4 1 ( 2 ) Ä , α = 7 6 . 3 8 ( 1 ) ° , β = 6 7 . 4 2 ( 1 ) , γ = 7 1 . 6 1 ( 1 ) ° , V= 6 7 6 . 8 Ä3, Z = 1, R&(F) = 0 . 0 4 2 ,
wR(F2) = 0 . 1 1 6 , 7 = 2 9 3 K .
Source of material
T h e t i t l e c o m p o u n d w a s p r e p a r e d b y r e d u c t i o n o f t h e c o r r e s - p o n d i n g i s o x a z o l i n e [ 1 - 7 ] u s i n g L i A l H U . A f t e r p r o t e c t i o n o f t h e a m i n e a s c a r b a m a t e , t h e r e s u l t i n g N - B o c p r o t e c t e d a m i n o d i o l w a s d i m e s y l a t e d . C r y s t a l l i z a t i o n f r o m p e t r o l e u m e t h e r / e t h y l a c e t a t e ( 4 : 1 ) f u r n i s h e d t h e t i t l e c o m p o u n d i n t h e f o r m o f c o l o u r l e s s c r y s - t a l s ( m p 4 1 3 К - 4 1 4 Κ , [ α ] * = - 3 5 . 1 , с = 1 . 2 7 , С Н С Ь ) .
Discussion
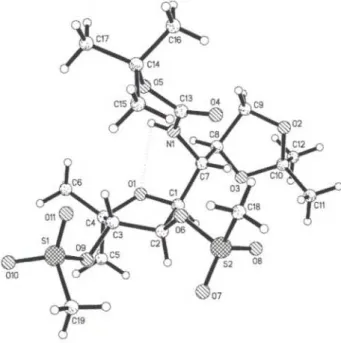
A w e a k i n t r a m o l e c u l a r h y d r o g e n b o n d i s o b s e r v e d b e t w e e n N 1 — H I and O l w i t h a H l — O l d i s t a n c e o f 2 . 5 2 Ä a n d a n N l - H l - O l a n g l e o f 9 5 . 1 ° , b u i l d i n g a five-membered r i n g ( N 1 , H l , O l , C I , C 7 ) w i t h e n v e l o p e c o n f o r m a t i o n . A d d i t i o n a l l y , w e find a n i n t e r m o l e c u l a r h y d r o g e n b o n d b e t w e e n N 1 — H I a n d 0 7 o f o n e o f the t w o s u l f o n y l o x y m o i e t i e s w i t h a H l — 0 7 d i s t a n c e o f 2 . 3 2 Ä a n d a n N 1 - H 1 - 0 7 a n g l e o f 1 6 0 . 7 ° .
Table 1. Data collection and handling.
Crystal: colourless block, size 0.4 χ 0.6 χ 1.75 mm
Wavelength: M o Ka radiation (0.71073 A)
μ: 2.48 cm"1
Diffractometer, scan mode: Nicolet P3, Wyckoff
20max: 58°
N(hkl)measured, JVfMOunique: 3 8 3 1 , 3 8 3 1 Criterion for /0bs, N(hkl)gt: /o b s > 2 of/obs), 3648
N(param)K fined: 309
Programs: SHELXS-86 [8], SHELXL-93 [9]
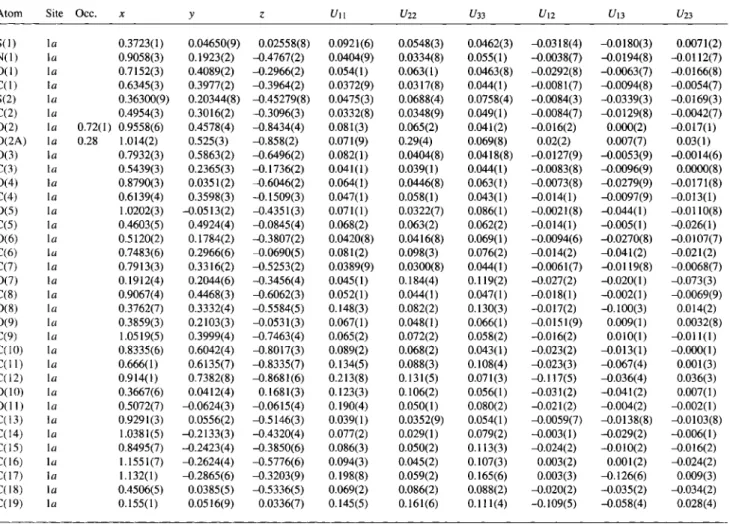
Table 2. Atomic coordinates and displacement parameters (in Ä2).
Atom Site X У Ζ t/,S„
H ( l ) 1 a 0.9613(3) 0.1965(2) -0.4215(2) 0.051 H(1 A) 1 α 0.5699(3) 0.4993(2) -0.4285(2) 0.047 H(2) la 0.3708(3) 0.3666(2) -0.2859(3) 0.047 H(3) 1 a 0.6404(3) 0.1422(3} -0.1883(2) 0.053 H(5A) 1 a 0.3785(5) 0.5292(4) - 0 . 1 3 8 9 ( 4 ) 0.100 H(5B) 1 a 0.5111(5) 0.5725(4) - 0 . 0 8 6 3 ( 4 ) 0.100 H(5C) la 0.3948(5) 0.4589(4) 0.0132(4) 0.100 H(6A) 1 a 0.8424(6) 0.2138(6) -0.1137(5) 0.121 H(6B) 1 a 0.6854(6) 0.2612(6) 0.0289(5) 0.121 H(6C) 1 a 0.8018(6) 0.3748(6) -0.0706(5) 0.121 H(7) la 0.7416(3) 0.3101(2) - 0 . 5 9 0 3 ( 2 ) 0.046 H(8) 1 a 0.9630(4) 0.4643(3) -0.5439(3) 0.060 H(9A) 1 a 1.1534(5) 0.4449(4) - 0 . 7 7 2 4 ( 4 ) 0.090 H(9B) 1 a 1.0969(5) 0.2905(4) - 0 . 7 4 0 8 ( 4 ) 0.090 H(11A) la 0.623(1) 0.5238(7) - 0 . 7 8 7 3 ( 7 ) 0.158 H(11B) 1 a 0.695(1) 0.6215(7) -0.9356(7) 0.158 H(11C) la 0.572(1) 0.7011(7) - 0 . 7 9 7 8 ( 7 ) 0.158 H(12A) la 1.021(1) 0.7231(8) -0.8426(6) 0.199 H(12B) la 0.826(1) 0.8284(8) - 0 . 8 3 3 1 ( 6 ) 0.199 H(12C) la 0.948(1) 0.7488(8) -0.9710(6) 0.199 H(15A) la 0.7776(7) -0.2102(4) -0.2920(6) 0.131 H(15B) la 0.8618(7) - 0 . 3 4 8 7 ( 4 ) -0.3797(6) 0.131 H(15C) l a 0.7904(7) -0.1863(4) -0.4536(6) 0.131 H(16A) la 1.0922(7) -0.2143(4) -0.6454(6) 0.143 H(16B) l a 1.1797(7) -0.3705(4) -0.5712(6) 0.143 H(16C) l a 1.2680(7) -0.2336(4) -0.6091(6) 0.143 H(17A) l a 1.054(1) -0.2529(6) -0.2290(9) 0.201 H(17B) l a 1.244(1) -0.2578(6) -0.3497(9) 0.201 H(17C) l a 1.156(1) -0.3948(6) -0.3118(9) 0.201 H(18A) l a 0.5674(5) 0.0406(5) -0.6060(5) 0.112 H(18B) l a 0.3687(5) 0.0316(5) - 0 . 5 7 7 4 ( 5 ) 0.112 H(18C) l a 0.4642(5) - 0 . 0 4 7 9 ( 5 ) -0.4622(5) 0.112 H(19A) l a 0.067(1) 0.1261(9) 0.0932(7) 0.188 H(19B) l a 0.131(1) -0.0463(9) 0.0737(7) 0.188 H(19C) l a 0.148(1) 0.0777(9) - 0 . 0 6 1 7 ( 7 ) 0.188
* Correspondence author (e-mail: jager.ioc@po.uni-stuttgart.de)
130
C 1 9 H 3 5 N 0 1 1 S 2Table 3. Atomic coordinates and displacement parameters (in Ä2).
Atom Site Occ. X У г ί / l l C/22 C/33 ί/12 С/13 С/23
S ( l ) l a 0.3723(1) 0.04650(9) 0.02558(8) 0.0921(6) 0.0548(3) 0.0462(3) -0.0318(4) - 0 . 0 1 8 0 ( 3 ) 0.0071(2) N ( l ) 1 a 0.9058(3) 0.1923(2) - 0 . 4 7 6 7 ( 2 ) 0.0404(9) 0.0334(8) 0.055(1) -0.0038(7) -0.0194(8) -0.0112(7) 0 ( 1 ) la 0.7152(3) 0.4089(2) - 0 . 2 9 6 6 ( 2 ) 0.054(1) 0.063(1) 0.0463(8) -0.0292(8) - 0 . 0 0 6 3 ( 7 ) -0.0166(8) C ( l ) 1 a 0.6345(3) 0.3977(2) - 0 . 3 9 6 4 ( 2 ) 0.0372(9) 0.0317(8) 0.044(1) -0.0081(7) -0.0094(8) -0.0054(7) S(2) la 0.36300(9) 0.20344(8) -0.45279(8) 0.0475(3) 0.0688(4) 0.0758(4) -0.0084(3) -0.0339(3) -0.0169(3) C(2) la 0.4954(3) 0.3016(2) - 0 . 3 0 9 6 ( 3 ) 0.0332(8) 0.0348(9) 0.049(1) -0.0084(7) -0.0129(8) -0.0042(7) 0 ( 2 ) la 0.72(1) 0.9558(6) 0.4578(4) - 0 . 8 4 3 4 ( 4 ) 0.081(3) 0.065(2) 0.041(2) -0.016(2) 0.000(2) -0.017(1) 0 ( 2 A ) la 0.28 1.014(2) 0.525(3) - 0 . 8 5 8 ( 2 ) 0.071(9) 0.29(4) 0.069(8) 0.02(2) 0.007(7) 0.03(1) 0 ( 3 ) la 0.7932(3) 0.5863(2) - 0 . 6 4 9 6 ( 2 ) 0.082(1) 0.0404(8) 0.0418(8) -0.0127(9) -0.0053(9) -0.0014(6) C(3) la 0.5439(3) 0.2365(3) - 0 . 1 7 3 6 ( 2 ) 0.041(1) 0.039(1) 0.044(1) -0.0083(8) -0.0096(9) 0.0000(8) 0 ( 4 ) 1 a 0.8790(3) 0.0351(2) - 0 . 6 0 4 6 ( 2 ) 0.064(1) 0.0446(8) 0.063(1) -0.0073(8) -0.0279(9) -0.0171(8) C(4) la 0.6139(4) 0.3598(3) - 0 . 1 5 0 9 ( 3 ) 0.047(1) 0.058(1) 0.043(1) -0.014(1) -0.0097(9) -0.013(1) 0 ( 5 ) la 1.0202(3) - 0 . 0 5 1 3 ( 2 ) -0.4351(3) 0.071(1) 0.0322(7) 0.086(1) -0.0021(8) -0.044(1) -0.0110(8)
C(5) la 0.4603(5) 0.4924(4) -0.0845(4) 0.068(2) 0.063(2) 0.062(2) -0.014(1) -0.005(1) -0.026(1)
0 ( 6 ) la 0.5120(2) 0.1784(2) - 0 . 3 8 0 7 ( 2 ) 0.0420(8) 0.0416(8) 0.069(1) -0.0094(6) -0.0270(8) -0.0107(7)
C(6) la 0.7483(6) 0.2966(6) -0.0690(5) 0.081(2) 0.098(3) 0.076(2) -0.014(2) -0.041(2) -0.021(2)
C(7) la 0.7913(3) 0.3316(2) - 0 . 5 2 5 3 ( 2 ) 0.0389(9) 0.0300(8) 0.044(1) -0.0061(7) -0.0119(8) -0.0068(7) 0 ( 7 ) la 0.1912(4) 0.2044(6) - 0 . 3 4 5 6 ( 4 ) 0.045(1) 0.184(4) 0.119(2) -0.027(2) -0.020(1) -0.073(3) C(8) la 0.9067(4) 0.4468(3) -0.6062(3) 0.052(1) 0.044(1) 0.047(1) -0.018(1) - 0 . 0 0 2 ( 1 ) -0.0069(9) 0 ( 8 ) la 0.3762(7) 0.3332(4) - 0 . 5 5 8 4 ( 5 ) 0.148(3) 0.082(2) 0.130(3) -0.017(2) -0.100(3) 0.014(2) 0 ( 9 ) la 0.3859(3) 0.2103(3) -0.0531(3) 0.067(1) 0.048(1) 0.066(1) -0.0151(9) 0.009(1) 0.0032(8) C(9) la 1.0519(5) 0.3999(4) - 0 . 7 4 6 3 ( 4 ) 0.065(2) 0.072(2) 0.058(2) -O.Ol 6(2) 0.010(1) -0.011(1) C(10) la 0.8335(6) 0.6042(4) - 0 . 8 0 1 7 ( 3 ) 0.089(2) 0.068(2) 0.043(1) -0.023(2) - 0 . 0 1 3 ( 1 ) -0.000(1) C ( l l ) la 0.666(1) 0.6135(7) - 0 . 8 3 3 5 ( 7 ) 0.134(5) 0.088(3) 0.108(4) -0.023(3) - 0 . 0 6 7 ( 4 ) 0.001(3) C(12) la 0.914(1) 0.7382(8) - 0 . 8 6 8 1 ( 6 ) 0.213(8) 0.131(5) 0.071(3) -0.117(5) - 0 . 0 3 6 ( 4 ) 0.036(3) 0 ( 1 0 ) la 0.3667(6) 0.0412(4) 0.1681(3) 0.123(3) 0.106(2) 0.056(1) -0.031(2) - 0 . 0 4 1 ( 2 ) 0.007(1) O ( l l ) la 0.5072(7) - 0 . 0 6 2 4 ( 3 ) - 0 . 0 6 1 5 ( 4 ) 0.190(4) 0.050(1) 0.080(2) -0.021(2) - 0 . 0 0 4 ( 2 ) -0.002(1) C(13) la 0.9291(3) 0.0556(2) - 0 . 5 1 4 6 ( 3 ) 0.039(1) 0.0352(9) 0.054(1) -0.0059(7) -0.0138(8) -0.0103(8) C(14) la 1.0381(5) - 0 . 2 1 3 3 ( 3 ) - 0 . 4 3 2 0 ( 4 ) 0.077(2) 0.029(1) 0.079(2) -0.003(1) - 0 . 0 2 9 ( 2 ) -0.006(1) C(15) la 0.8495(7) - 0 . 2 4 2 3 ( 4 ) - 0 . 3 8 5 0 ( 6 ) 0.086(3) 0.050(2) 0.113(3) -0.024(2) - 0 . 0 1 0 ( 2 ) -0.016(2) с о 6) 1 a 1.1551(7) - 0 . 2 6 2 4 ( 4 ) - 0 . 5 7 7 6 ( 6 ) 0.094(3) 0.045(2) 0.107(3) 0.003(2) 0.001(2) -0.024(2) C(17) 1 a 1.132(1) - 0 . 2 8 6 5 ( 6 ) - 0 . 3 2 0 3 ( 9 ) 0.198(8) 0.059(2) 0.165(6) 0.003(3) -0.126(6) 0.009(3) CO 8) la 0.4506(5) 0.0385(5) - 0 . 5 3 3 6 ( 5 ) 0.069(2) 0.086(2) 0.088(2) -0.020(2) - 0 . 0 3 5 ( 2 ) -0.034(2)
CO 9) la 0.155(1) 0.0516(9) 0.0336(7) 0.145(5) 0.161(6) 0.111(4) -0.109(5) -0.058(4) 0.028(4)
Acknowledgments. W e are grateful to the Landesgraduiertenförderung Baden-Württemberg (doctoral fellowship to P. J. Zimmermann). For finan- cial support of this work we thank Volkswagenstiftung (Hannover), Fonds der Chemischen Industrie, and Bayer AG, Wuppertal. I. Blanarikova gratefully acknowledges a grant from Volkswagenstiftung (Hannover) for a research stay at Stuttgart.
References
1. Jäger, V.; Müller, I.: Synthesis of Amino Sugars via Isoxazolines. Nitrile Oxide - Furan Adducts as Key Intermediates in the Isoxazoline Route To- w a r d s Novel A m i n o S u g a r D e r i v a t i v e s . T e t r a h e d r o n 4 1 ( 1 9 8 5 ) 3519-3528.
2. Zimmermann, P. J.: Furo[2,3-iflisoxazoline - Schlüsselbausteine in der Synthese von L-(+)-Furanomycin und Desoxynojirimycin - Homologen.
Dissertation, Universität Stuttgart, in preparation.
3. Zimmermann, P. J.; Blanarikova, I.; Jäger, V.: A General Approach to L-(+)-Furanomycin, Some Stereoisomers, and Analogues Using Furo- isoxazoline Intermediates. Angew. Chem., in print.
4. Jäger, V.; Leibold, Т.; Müller, R.; Svete, J.; Z i m m e r m a n n , P. J.:
Furo[2,3-d]isoxazolines - Key Intermediates in the Synthesis of Bio- logically Active Compounds. Book of abstracts, 17 th ICHC Vienna 1999.
5. Müller, I.; Jäger, V.: Synthesis of Amino Sugars via Isoxazolines. The Concept and One Application: Nitrile Oxide/Furan Adducts. Tetrahedron Lett. 2 3 (1982) 4777-4780.
6. Jäger, V.; Grund, Η.; Büß, V.; Schwab, W.; Müller, I.; Schohe, R.; Franz, R.; Ehrler, R.: Isoxazolines - Key Intermediates for Syntheses of Some Naturally Occuring Amino Compounds. Bull. Soc. Chim. Belg. 92 (1983)
1039-1054.
7. Müller, R.; Leibold, Т.; Pätzel, Μ.; Jäger, V.: Eine neue Synthese von 1,3,4-Tridesoxy-1,4-iminoglyciten mit variabler Kettenlänge durch (C3 + Cn) - V e r k n ü p f u n g von Allylhalogeniden und G l y c o n o n i t r i l o x i d e n . Angew. Chem. 106 (1994) 1305-1308; Angew. Chem. Int. Ed. Engl. 33 (1994) 1295-1298.
8. Sheldrick, G. M.: Phase Annealing in SHELX-90: Direct Methods for Larger Structures. Acta Crystallogr. A46 (1990) 467-473.
9. Sheldrick, G. M.: SHELXL-93. Program for the Refinement of Crystal Structures. University of Göttingen, Germany 1993.