Zeitschrift fur Kristallographie - New Crystal Structures 212, 203-204
© by R. Oldenbourg Verlag, München 1997
203
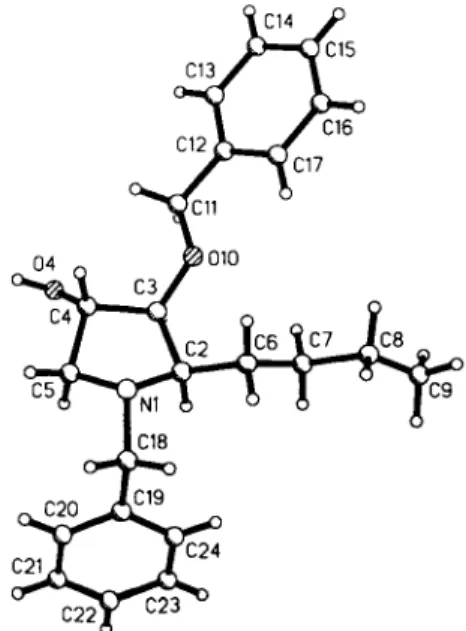
Crystal structure of (2/?,35,4S)-iV,3-0-dibenzyl-2-butyl-3,4-dihydroxy·
pyrrolidine,
C 2 2 H 2 9 N O 2S. Henkel, O. Schwardt and V. Jäger
Universität Stuttgart. Institut für Organische Chemie, Pfaffenwaldring 55, D-70569 Stuttgart, Germany
Received August 7, 1996, CSD-No. 402588
Source of material: The title compound (see ref. 1) was prepared by Mitsunobu cyclization of (2S,3S,4/?)-3-0-benzyl-4-benzyl- amino-1,2,3-octantriol (see refs. 1,2) with triphenylphosphine and diethyl azodicarboxylate in pyridine. The crude product was puri- fied by silica gel chromatography with petroleum ether/diethyl ether 3:7 as eluent. After crystallization from heptane/ether the pyrrolidine was isolated in 76 % yield as colorless needles with mp 341-342 K.
The title compound belongs to a series of franj-dihydroxypyrro- lidines such as anisomycin, a known antibiotic which was recently found to have strong anti-tumor activities (see ref. 3).
C 2 2 H 2 9 N O 2 , monoclinic, P\2\\ (No. 4), a =12.004(3) Â,
b =7.301(2) Â, c =12.663(3) À, β =112.96(2)°, V=1021.9Â3, Z=2, R(F) =0.057, Ry/F2) =0.123.
Table 1. Parameters used for the X-ray data collection
Crystal: colorless needles, size 0.2 χ 0.25 χ 1.5 mm Wavelength: Mo Ka radiation (0.71073 λ )
μ: 0.70 cm"1
Diffractometer: Nicolet P3
Scan mode: Wyckoff
Τ measurement' 293 Κ
2θπηχ: 50°
N(A¿/)umfu<: 1915 Criterion for l0: Io >2 σ(/0)
N(param)rcfme<f. 231
Programs: SHELXS-86, SHELXL-93
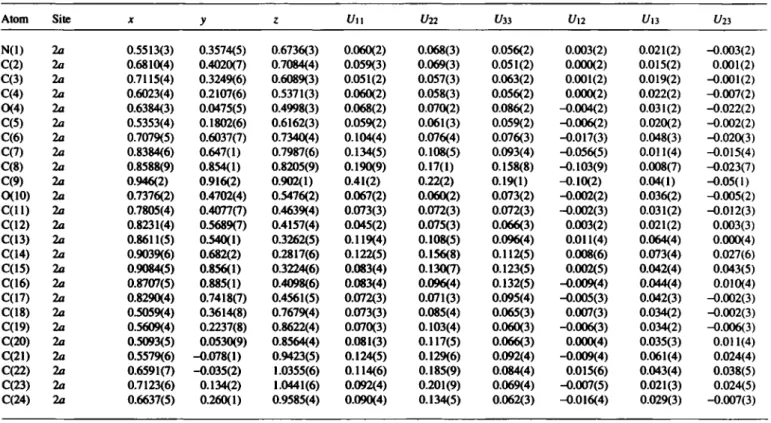
Table 2. Final atomic coordinates and displacement parameters (in A2)
Atom Site χ y ζ i/i»
2a 2a 2a 2a
H(2) H(3) H(4) H(4A)
H(5A) 2a H(5B) 2a H(6A) 2a H(6B) 2a H(7A) 2a H(7B)
H(8A) H(8B)
H(9A) 2a
H(9B) 2a
H(9C) 2a
H(11A) 2a H(11B) 2a H(13)
2a
2a 2a
H(14) H(15) H(16) H(17)
2a 2a 2a 2a 2a
H(18A) la H(18B) 2a H(20) H(21) H(22) H(23) H(24)
2a 2a 2a 2a 2a
0.7270(4) 0.7825(4) 0.5510(4) 0.576(4) 0.4504(4) 0.5715(4) 0.6794(5) 0.6626(5) 0.8846(6) 0.8675(6) 0.8616(9) 0.7868(9) 0.943(2)
1.020(2) 0.944(2) 0.7162(4) 0.8467(4) 0.8574(5) 0.9301(6) 0.9365(5) 0.8732(5) 0.8045(4) 0.4190(4) 0.5201(4) 0.4396(5) 0.5219(6) 0.6924(7) 0.7814(6) 0.7010(5)
0.3324(7) 0.2449(6) 0.2820(6) 0.002(7) 0.1532(6) 0.0821(6) 0.6704(7) 0.6471(7) 0.604(1) 0.583(1) 0.907(1) 0.902(1) 1.047(2) 0.877(2) 0.872(2) 0.3447(7) 0.3223(7) 0.422(1) 0.660(2) 0.952(1) 1.003(1) 0.7649(7) 0.3422(8) 0.4828(8) 0.0244(9) -0.193(1) -0.120(2) 0.162(2) 0.374(1)
0.7780(4) 0.6406(3) 0.4702(3) 0.449(4) 0.5730(3) 0.6704(3) 0.6621(4) 0.7779(4) 0.7555(6) 0.8716(6) 0.7513(9) 0.8278(9) 0.900(1) 0.895(1) 0.972(1) 0.4028(4) 0.4986(4) 0.2963(5) 0.2228(6) 0.2915(6) 0.4387(6) 0.5161(5) 0.7346(4) 0.8017(4) 0.7929(4) 0.9359(5) 1.0939(6) 1.1081(6) 0.9654(4)
0.074 0.070 0.070 0.07(2) 0.072 0.072 0.097 0.097 0.146 0.146 0.229 0.229 0.445 0.445 0.445 0.086 0.086
0.121
0.146 0.134 0.123 0.091 0.087 0.087 0.103 0.131 0.152 0.149 0.114
204
(2Ä,35,45)-Ar,3-0-dibenzyl-2-butyl-3,4-dihydroxy-pyrrolidineTable 3. Final atomic coordinates and displacement parameters (in Â2)
Atom Site X y ζ l/ii Un C/33 Ul2 U,3 Un
N(l) 2a 0.5513(3) 0.3574(5) 0.6736(3) 0.060(2) 0.068(3) 0.056(2) 0.003(2) 0.021(2) -0.003(2) C(2) 2a 0.6810(4) 0.4020(7) 0.7084(4) 0.059(3) 0.069(3) 0.051(2) 0.000(2) 0.015(2) 0.001(2) C(3) 2a 0.7115(4) 0.3249(6) 0.6089(3) 0.051(2) 0.057(3) 0.063(2) 0.001(2) 0.019(2) -0.001(2) C(4) 2a 0.6023(4) 0.2107(6) 0.5371(3) 0.060(2) 0.058(3) 0.056(2) 0.000(2) 0.022(2) -0.007(2) 0(4) 2a 0.6384(3) 0.0475(5) 0.4998(3) 0.068(2) 0.070(2) 0.086(2) -0.004(2) 0.031(2) -0.022(2) C(5) 2a 0.5353(4) 0.1802(6) 0.6162(3) 0.059(2) 0.061(3) 0.059(2) -0.006(2) 0.020(2) -0.002(2) C(6) 2a 0.7079(5) 0.6037(7) 0.7340(4) 0.104(4) 0.076(4) 0.076(3) -0.017(3) 0.048(3) -0.020(3) C(7) 2a 0.8384(6) 0.647(1) 0.7987(6) 0.134(5) 0.108(5) 0.093(4) -0.056(5) 0.011(4) -0.015(4) C(8) 2a 0.8588(9) 0.854(1) 0.8205(9) 0.190(9) 0.17(1) 0.158(8) -0.103(9) 0.008(7) -0.023(7)
C(9) 2a 0.946(2) 0.916(2) 0.902(1) 0.41(2) 0.22(2) 0.19(1) -0.10(2) 0.04(1) -0.05(1)
0(10) 2a 0.7376(2) 0.4702(4) 0.5476(2) 0.067(2) 0.060(2) 0.073(2) -0.002(2) 0.036(2) -0.005(2) C ( l l ) 2a 0.7805(4) 0.4077(7) 0.4639(4) 0.073(3) 0.072(3) 0.072(3) -0.002(3) 0.031(2) -0.012(3) C(12) 2a 0.8231(4) 0.5689(7) 0.4157(4) 0.045(2) 0.075(3) 0.066(3) 0.003(2) 0.021(2) 0.003(3) C(13) 2a 0.8611(5) 0.540(1) 0.3262(5) 0.119(4) 0.108(5) 0.096(4) 0.011(4) 0.064(4) 0.000(4) C(14) 2a 0.9039(6) 0.682(2) 0.2817(6) 0.122(5) 0.156(8) 0.112(5) 0.008(6) 0.073(4) 0.027(6) C(15) 2a 0.9084(5) 0.856(1) 0.3224(6) 0.083(4) 0.130(7) 0.123(5) 0.002(5) 0.042(4) 0.043(5) C(16) 2a 0.8707(5) 0.885(1) 0.4098(6) 0.083(4) 0.096(4) 0.132(5) -0.009(4) 0.044(4) 0.010(4) C(17) 2a 0.8290(4) 0.7418(7) 0.4561(5) 0.072(3) 0.071(3) 0.095(4) -0.005(3) 0.042(3) -0.002(3) C(18) 2a 03059(4) 0.3614(8) 0.7679(4) 0.073(3) 0.085(4) 0.065(3) 0.007(3) 0.034(2) -0.002(3) C(19) 2a 0.5609(4) 0.2237(8) 0.8622(4) 0.070(3) 0.103(4) 0.060(3) -0.006(3) 0.034(2) -0.006(3) C(20) 2a 0.5093(5) 0.0530(9) 0.8564(4) 0.081(3) 0.117(5) 0.066(3) 0.000(4) 0.035(3) 0.011(4) C(21) 2a 0.5579(6) -0.078(1) 0.9423(5) 0.124(5) 0.129(6) 0.092(4) -0.009(4) 0.061(4) 0.024(4) C(22) 2a 0.6591(7) -0.035(2) 1.0355(6) 0.114(6) 0.185(9) 0.084(4) 0.015(6) 0.043(4) 0.038(5) C(23) 2a 0.7123(6) 0.134(2) 1.0441(6) 0.092(4) 0.201(9) 0.069(4) -0.007(5) 0.021(3) 0.024(5) C(24) 2a 0.6637(5) 0.260(1) 0.9585(4) 0.090(4) 0.134(5) 0.062(3) -0.016(4) 0.029(3) -0.007(3)
References
1. Veith, U.; Schwardt, O.; Jäger, V: Concise synthesis of deacetylaniso- mycin and 2-substituted analogues from N,2-0-dibenzyl-L-threose imine. Synlett. In print.
2. Veith, U.: Stereoselektiver Aufbau von Aminodiolen und -triolen aus optisch aktiven Aldehyden - Synthese enantiomerenreiner, stickstoffhal- tiger Natur- und Wirkstoffe. Dissertation, University of Stuttgart, Ger- many 1995.
3. Veith, U.; Leurs, S.; Jäger, V.: Auxiliary-controlled diastereoselection by N-{ 1 -phenylethy 1) in Grignard additions to 2-0-benzyl-glyceraldehyde
¡mines. J. Chem. Soc. Chem. Commun. (19%) 329-330.
4. Hosoya, Y.; Kameyama, T.; Naganawa, H.; Okami, Y.; Takeuchi, T.:
Anisomycin and new congeners active against human tumor cell lines. J.
AntibioL 46 (1993) 1300-1302.
5. Sheldrick, G. M.: SHELX-86. Program for the solution of crystal structures. University of Göttingen, Germany 1986.
6. Sheldrick, G. M.: SHELXL-93, a program for refining crystal structures.
University of Göttingen, Germany 1993.