ζ . Kristallogr. NCS 213 (1998) 7 0 1 - 7 0 2
701
© by R. Oldenbourg Verlag, München
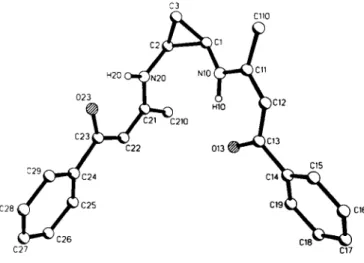
Crystal structure of cw-iV^'-bis(l-methyl-3-phenyl-l-propen-3-onyl)-l,2- cyclopropanediamine, C3H6N2(CioH90)2
K. Peters, E.-M. Peters
Max-Planck-Institut für Festkörperforschung, Heisenbergstraße I. D-70506 Stuttgart. Germany R. Reinhardt and H. Quast
Universität Würzburg, Institut für Organische Chemie, Am Hubland, D-97074 Würzburg, Germany Received February 26, 1998, CSD-No. 409244
Source of material: The title compound was prepared, according to ref. 1, from cif-l,2-cyclopropanediammonium dibromide and l-phenylbutane-l,3-dione (2 equiv.) in a mixture of methanol and acetic acid (ca. 4:1) in the presence of a large excess of sodium acetate by heating under reflux for 3 min. Addition of sodium Perchlorate (6 equiv.) to the hot mixttu-e and cooling yielded a precipitate which was recrystalUzed from methanol and subsequently treated with concentrated aqueous potassium hydroxide and ether to afford a yellow oil after evaporation of the solvent. Trituration with methanol and recrystallization furnished colorless crystals, mp 391 К - 3 9 2 К.
The molecular structure of the title compound is characterized by two ß-aminoenone moieties which adopt the Zconfigivation and different orientations relative to the three-membered ting. The Zconfíguration allows the formation of an intramolecular hydrogen bridge (NlO-HlO- 0 1 3 and N 2 0 - H 2 0 - 0 2 3 ) . The title compound is a tetradentate ligand for transition metal ions, e.g. nickel(n), which readily affords a diamagnetic complex, mp 504.5 К - 505 К, in almost quantitative yield.
C23H24N2O2, orthorhombic, P2i2i2i (No. 19), a =16.197(3) Â,
¿>=19.766(4) Â, с =6.031(1) Â, ν = 1 9 3 0 . 8 Α Λ Ζ =4, Ä^F) =0.043, Rv4F) =0.042.
Table 1. Parameters used for the X-ray data collection
Crystal: colorless prism, 0.45 χ 0.3 χ 0.65 mm Wavelength: Mo Ka radiation (0.71073 Â)
μ: 0.80 cm '
Diñiractometer: SYNTEXP3
Scan mode: ω
Tmgasuremen/' 293 К
55°
2335 Criterion for Fo: Fo>3o(Fo)
1Я(рагат)гфаГ. 252
Program: SHELXTL-plus
Table 2. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X У г í/iso
H(l) 4a 0.8921(2) 0.2356(1) -0.0828(5) 0.08 H(2) 4a 1.0494<2) 0.2002(1) -0.2603(5) 0.08 H(3A) 4a 0.9821(2) 0.1360(1) 0.0814(6) 0.08 H(3B) 4a 1.0233(2) 0.1926(1) 0.2261(6) 0.08 H(10) 4a 0.956(2) 0.341(1) -0.009(6) 0.09(1) H(12) 4a 0.8466(2) 0.3933(1) 0.4244(5) 0.08 H(15) 4a 0.8329(2) 0.4941(1) 0.5248(6) 0.08 H(16) 4a 0.7744(2) 0.6015(2) 0.5708(6) 0.08 H(17) 4a 0.7741(2) 0.6788(2) 0.2796(6) 0.08 H(I8) 4a 0.8379(2) 0.6509(1) -0.0549(6) 0.08 H(19) 4a 0.8989(2) 0.5446(1) -0.1019(5) 0.08 H(20) 4a 1.132(2) 0.261(2) 0.033(6) 0.09(1) H(22) 4a 1.1901(2) 0.3931(1) -0.2848(5) 0.08 H(25) 4a 1.2940(2) 0.4507(1) -0.2579(5) 0.08 H(26) 4a 1.4058(2) 0.5234(1) -0.1963(6) 0.08 H(27) 4a 1.4738(2) 0.5233(1) 0.1455(6) 0.08 H(28) 4a 1.4295(2) 0.4518(1) 0.4266(6) 0.08 H(29) 4a 1.3196(2) 0.3770(1) 0.3672(5) 0.08 H(llA) 4a 0.8173(2) 0.2807(1) 0.4779(6) 0.08 H(llB) 4a 0.8918(2) 0.2328(1) 0.4244(6) 0.08 H(llC) 4a 0.8159(2) 0.2360(1) 0.2632(6) 0.08 H(21A) 4a 1.0875(2) 0.3577(2) -0.5177(6) 0.08 H(21B) 4a 1.0112(2) 0.3251(2) -0.3992(6) 0.08 H(21C) 4a 1.0750(2) 0.2791(2) -0.5237(6) 0.08
702
C3H6N2(CI0H9O)2 Table 3. Final atomic coordinates and displacement parameters (in Â^)Atom Site X ζ i/ll t/22 Í/33 Í/I2 Í/13 t/23
C d ) 4a 0.9479(2) 0.2345(1) -0.0289(5) 0.047(1) 0.050(1) 0.066(2) -0.006(1) -0.003(2) -0.003(1) C(2) 4a 1.0301(2) 0.2150(1) -0.1178(5) 0.051(1) 0.049(1) 0.066(2) -0.003(1) 0.001(2) -0.009(1) C(3) 4a 0.9986(2) 0.1827(1) 0.0851(6) 0.061(2) 0.044(1) 0.082(2) -0.007(1) -0.005(2) 0.002(2) N(IO) 4a 0.9403(1) 0.3012(1) 0.0661(4) 0.053(1) 0.045(1) 0.060(2) -0.002(1) 0.004(1) 0.000(1) C ( l l ) 4a 0.8908(2) 0.3177(1) 0.2362(5) 0.041(1) 0.059(1) 0.052(2) -0.003(1) -0.003(1) 0.006(1) C(12) 4a 0.8769(2) 0.3840(1) 0.2911(5) 0.046(1) 0.056(1) 0.049(2) 0.000(1) 0.003(1) 0.001(1) C(13) 4a 0.9049(2) 0.4392(1) 0.1619(5) 0.045(1) 0.056(1) 0.057(2) -0.003(1) 0.006(1) -0.004(1) 0(13) 4a 0.9533(1) 0.43261(9) 0.0019(4) 0.083(2) 0.056(1) 0.084(2) -0.002(1) 0.041(1) 0.000(1) C(14) 4a 0.8706(2) 0.5084(1) 0.2052(5) 0.044(1) 0.050(1) 0.055(2) -0.008(1) 0.002(1) -0.006(1) C(15) 4a 0.8340(2) 0.5260(1) 0.4047(6) 0.068(2) 0.061(2) 0.059(2) -0.008(1) 0.011(2) -0.007(1) C(16) 4a 0.7990(2) 0.5895(2) 0.4314(6) 0.067(2) 0.070(2) 0.070(2) -0.003(2) 0.011(2) -0.020(2) C(17) 4a 0.7997(2) 0.6354(2) 0.2614(6) 0.053(2) 0.055(2) 0.095(3) 0.001(1) -0.005(2) -0.019(2) C(18) 4a 0.8366(2) 0.6187(1) 0.0642(6) 0.060(2) 0.054(2) 0.078(2) -0.003(1) -0.007(2) 0.000(2) C(19) 4a 0.8726(2) 0.5556(1) 0.0361(5) 0.054(2) 0.057(1) 0.059(2) -0.008(1) 0.004(2) -0.003(1) N(20) 4a 1.0992(1) 0.2611(1) -0.0919(5) 0.048(1) 0.055(1) 0.063(2) -0.004(1) -0.006(1) -0.001(1) C(2I) 4a 1.1177(2) 0.3125(1) -0.2274(5) 0.044(1) 0.049(1) 0.056(2) 0.003(1) -0.004(1) -0.004(1) C(22) 4a 1.1797(2) 0.3574(1) -0.1806(5) 0.046(1) 0.049(1) 0.055(2) -0.001(1) -0.004(1) 0.003(1) C(23) 4a 1.2292(2) 0.3543(1) 0.0124(5) 0.045(1) 0.046(1) 0.052(2) 0.001(1) -0.001(1) -0.001(1) 0(23) 4a 1.2212(1) 0.3092(1) 0.1557(4) 0.072(1) 0.072(1) 0.065(1) -0.021(1) -0.016(1) 0.018(1) C(24) 4a 1.2967(2) 0.4056(1) 0.0463(5) 0.044(1) 0.044(1) 0.051(2) 0.005(1) -0.003(1) -0.005(1) C(25) 4a 1.3221(2) 0.4500(1) -0.1177(5) 0.058(2) 0.051(1) 0.055(2) -0.004(1) -0.008(2) -0.004(1) C(26) 4a 1.3880(2) 0.4933(1) -0.0808(6) 0.066(2) 0.055(2) 0.071(2) -0.013(1) 0.001(2) -0.001(2) C(27) 4a 1.4279(2) 0.4935(1) 0.1206(6) 0.059(2) 0.057(2) 0.083(2) -0.011(1) -0.007(2) -0.017(2) C(28) 4a 1.4022(2) 0.4510(1) 0.2853(6) 0.058(2) 0.068(2) 0.063(2) 0.002(1) -0.013(2) -0.015(2) C(29) 4a 1.3369(2) 0.4069(1) 0.2506(5) 0.052(2) 0.057(1) 0.053(2) 0.002(1) -0.002(1) -0.006(1) C(llO) 4a 0.8504(2) 0.2618(1) 0.3614<6) 0.071(2) 0.064(2) 0.077(2) -0.005(2) 0.013(2) 0.008(2) C(210) 4a 1.0684(2) 0.3192(2) -0.4356(6) 0.076(2) 0.073(2) 0.070(2) -0.016(2) -0.021(2) 0.004(2)
References
1. Reinhardt, R.; Reaktionen von 1,2-Cyclopropandiaminen mit Carbonyl- verbindungen. Zur Reaktion von Malondialdehyd mit Nucleinsäurebasen.
Dissertation, Universität Würzburg, Germany 1985.
2. Sheldrick, G. M.: Program Package SHELXTL-plus. Release 4.1.
Siemens Analytical X-Ray Instruments Inc., Madison (WI53719), USA 1990.