Zeitschrift für Kristallographie - New Crystal Structures 2 1 2 , 4 2 1 - 4 2 2
© by R. Oldenbourg Verlag, München 1997
421
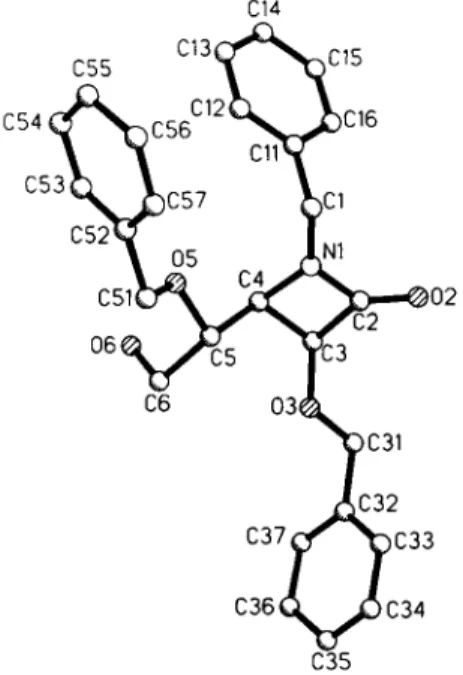
Crystal structure of (3S,4/?,l'^)-l-benzyl-3-benzyloxy-4-(l / -benzyloxy-2 / - hydroxyethyI)-azetidine-2-one, C26H27NO4
K. P e t e r s , E . - M . Peters
Max-Planck-Institut für Festkörperforschung. Heisenbergstraße 1, D-70506 Stuttgart, Germany T. F r a n z a n d V . J ä g e r
I n s t i t u t für Organische Chemie der Universität, Pfaffenwaldring 55, D-70569 Stuttgart, Germany
Received November 19, 1996, CSD-No. 402716
Table 1. Parameters used for the X-ray data collection
C35
Source of material: The title compound (alternative name: 2,4-di- 0 - b e n z y l - 3 - b e n z y I a m i n o - 3 - d e o x y - L - x y l o n o - 1 , 3 - l a c t a m ) was prepared by [2+2] cycloaddition from N,2-0-dibenzyl-glyceral- dimine (see réf. 1) with benzyloxyacetyl chloride in CH2CI2 using Et3N, followed by reaction with vinylmagnesium bromide in THF at 195 Κ (see refs. 2-5).
The Η atom at 0 ( 6 ) is rationally disordered. Therefore, it is not possible to define its location.
C26H27NO4, monoclinic, P\2\ 1 (No. 4), a =13.287(2) Â,
b =8.276(2) Â, c =11.720(3) Â, β =114.38(3)°, V=l 173.8 Â3, Z = 2 , R(F) =0.075, Rv/F) =0.059.
Crystal: colorless lath, size 0.5 χ 1.0 χ 0.3 mm Wavelength: Mo Ka radiation (0.71073 Â)
μ: 0.80 cm"1
Diffractometen Siemens P4
Scan mode: ω
Tmeasuremeni' 293 Κ
2θπαχ· 55°
Nía*/)«™,.«·: 4132
Criterion for F0: Fa > 3 a(Fo) N(param)r&ied: 280
Program: SHELXTL-plus
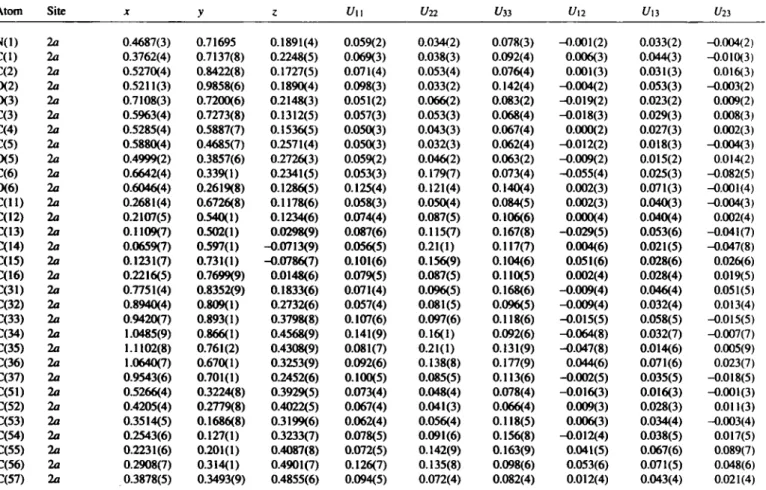
Table 2. Final atomic coordinates and displacement parameters (in Â2)
Atom Site X y ζ l/i»
H(1A) 2a 0.3910(4) 0.6343(8) 0.2895(5) 0.08 H(1B) 2a 0.3696(4) 0.8183(8) 0.2566(5) 0.08 H(3) 2a 0.6032(4) 0.7430(8) 0.0536(5) 0.08 H(4) 2a 0.4933(4) 0.5093(7) 0.0898(5) 0.08 H(5) 2a 0.6391(4) 0.5277(7) 0.3277(4) 0.08 H(6A) 2a 0.6911(4) 0.264(1) 0.3026(5) 0.08 H(6B) 2a 0.7256(4) 0.391(1) 0.2267(5) 0.08 H(12) 2a 0.2411(5) 0.471(1) 0.1954(6) 0.08 H(13) 2a 0.0727(7) 0.407(1) 0.0365(9) 0.08 H(14). . 2a -0.0047(7) 0.571(1) -0.1371(9) 0.08 H(15) 2a 0.0928(7) 0.799(1) -0.1513(7) 0.08 H(16) 2a 0.2591(5) 0.8664(9) 0.0087(6) 0.08 H(31A) 2a 0.7666(4) 0.8187(9) 0.0987(6) 0.08 H(31B) 2a 0.7521(4) 0.9429(9) 0.1919(6) 0.08 H(33) 2a 0.9003(7) 0.973(1) 0.4016(8) 0.08 H(34) 2a 1.0806(9) 0.926(1) 0.5337(9) 0.08 H(35) 2a 1.1869(8) 0.749(2) 0.4856(9) 0.08 H(36) 2a 1.1057(7) 0.588(1) 0.3066(9) 0.08 H(37) 2a 0.9212(6) 0.642(1) 0.1680(6) 0.08 H(51A) 2a 0.5721(4) 0.2282(8) 0.4055(5) 0.08 H(51B) 2a 0.5654(4) 0.4021(8) 0.4551(5) 0.08 H(53) 2a 0.3721(5) 0.1200(8) 0.2584(6) 0.08 H(54) 2a 0.2077(6) 0.047(1) 0.2668(7) 0.08 H(55) 2a 0.1539(6) 0.174(1) 0.4114(8) 0.08 H(56) 2a 0.2692(7) 0.367(1) 0.5495(7) 0.08 H(57) 2a 0.4355(5) 0.4275(9) 0.5430(6) 0.08
422
C26H27N04Table 3. Final atomic coordinates and displacement parameters (in Â2)
Atom Site X y ζ U M ί/22 ί/33 U12 U13 1/23
N(l) 2a 0.4687(3) 0.71695 0.1891(4) 0.059(2) 0.034(2) 0.078(3) -0.001(2) 0.033(2) -0.004(2) C(l) 2a 0.3762(4) 0.7137(8) 0.2248(5) 0.069(3) 0.038(3) 0.092(4) 0.006(3) 0.044(3) -0.010(3) C(2) 2a 0.5270(4) 0.8422(8) 0.1727(5) 0.071(4) 0.053(4) 0.076(4) 0.001(3) 0.031(3) 0.016(3) 0 ( 2 ) 2a 0.5211(3) 0.9858(6) 0.1890(4) 0.098(3) 0.033(2) 0.142(4) -0.004(2) 0.053(3) -0.003(2) 0 ( 3 ) 2a 0.7108(3) 0.7200(6) 0.2148(3) 0.051(2) 0.066(2) 0.083(2) -0.019(2) 0.023(2) 0.009(2) C(3) 2a 0.5963(4) 0.7273(8) 0.1312(5) 0.057(3) 0.053(3) 0.068(4) -0.018(3) 0.029(3) 0.008(3) C(4) 2a 0.5285(4) 0.5887(7) 0.1536(5) 0.050(3) 0.043(3) 0.067(4) 0.000(2) 0.027(3) 0.002(3) C(5) 2a 0.5880(4) 0.4685(7) 0.2571(4) 0.050(3) 0.032(3) 0.062(4) -0.012(2) 0.018(3) -0.004(3) CK5) 2a 0.4999(2) 0.3857(6) 0.2726(3) 0.059(2) 0.046(2) 0.063(2) -0.009(2) 0.015(2) 0.014(2) C(6) 2a 0.6642(4) 0.339(1) 0.2341(5) 0.053(3) 0.179(7) 0.073(4) -0.055(4) 0.025(3) -0.082(5) 0(6) 2a 0.6046(4) 0.2619(8) 0.1286(5) 0.125(4) 0.121(4) 0.140(4) 0.002(3) 0.071(3) -0.001(4) C ( U ) 2a 0.2681(4) 0.6726(8) 0.1178(6) 0.058(3) 0.050(4) 0.084(5) 0.002(3) 0.040(3) -0.004(3) C(12) 2a 0.2107(5) 0.540(1) 0.1234(6) 0.074(4) 0.087(5) 0.106(6) 0.000(4) 0.040(4) 0.002(4) C(13) 2a 0.1109(7) 0.502(1) 0.0298(9) 0.087(6) 0.115(7) 0.167(8) -0.029(5) 0.053(6) -0.041(7) C(14) 2a 0.0659(7) 0.597(1) -0.0713(9) 0.056(5) 0.21(1) 0.117(7) 0.004(6) 0.021(5) -0.047(8) C(15) 2a 0.1231(7) 0.731(1) -0.0786(7) 0.101(6) 0.156(9) 0.104(6) 0.051(6) 0.028(6) 0.026(6) C(16) 2a 0.2216(5) 0.7699(9) 0.0148(6) 0.079(5) 0.087(5) 0.110(5) 0.002(4) 0.028(4) 0.019(5) C(31) 2a 0.7751(4) 0.8352(9) 0.1833(6) 0.071(4) 0.096(5) 0.168(6) -0.009(4) 0.046(4) 0.051(5) C(32) 2a 0.8940(4) 0.809(1) 0.2732(6) 0.057(4) 0.081(5) 0.096(5) -0.009(4) 0.032(4) 0.013(4) C(33) 2a 0.9420(7) 0.893(1) 0.3798(8) 0.107(6) 0.097(6) 0.118(6) -0.015(5) 0.058(5) -0.015(5) C(34) 2a 1.0485(9) 0.866(1) 0.4568(9) 0.141(9) 0.16(1) 0.092(6) -0.064(8) 0.032(7) -0.007(7) C(35) 2a 1.1102(8) 0.761(2) 0.4308(9) 0.081(7) 0.21(1) 0.131(9) -0.047(8) 0.014(6) 0.005(9) C(36) 2a 1.0640(7) 0.670(1) 0.3253(9) 0.092(6) 0.138(8) 0.177(9) 0.044(6) 0.071(6) 0.023(7) C(37) 2a 0.9543(6) 0.701(1) 0.2452(6) 0.100(5) 0.085(5) 0.113(6) -0.002(5) 0.035(5) -0.018(5) C(51) 2a 0.5266(4) 0.3224(8) 0.3929(5) 0.073(4) 0.048(4) 0.078(4) -0.016(3) 0.016(3) -0.001(3) C(52) 2a 0.4205(4) 0.2779(8) 0.4022(5) 0.067(4) 0.041(3) 0.066(4) 0.009(3) 0.028(3) 0.011(3) C(53) 2a 0.3514(5) 0.1686(8) 0.3199(6) 0.062(4) 0.056(4) 0.118(5) 0.006(3) 0.034(4) -0.003(4) C(54) 2a 0.2543(6) 0.127(1) 0.3233(7) 0.078(5) 0.091(6) 0.156(8) -0.012(4) 0.038(5) 0.017(5) C(55) 2a 0.2231(6) 0.201(1) 0.4087(8) 0.072(5) 0.142(9) 0.163(9) 0.041(5) 0.067(6) 0.089(7) C(56) 2a 0.2908(7) 0.314(1) 0.4901(7) 0.126(7) 0.135(8) 0.098(6) 0.053(6) 0.071(5) 0.048(6) C(57) 2a 0.3878(5) 0.3493(9) 0.4855(6) 0.094(5) 0.072(4) 0.082(4) 0.012(4) 0.043(4) 0.021(4)
Acknowedgmenis. We are grateful to the Fonds der Chemischen Industrie and the Deutsche Forschungsgemeinschaft for financial support.
References
1. Franz, T.; Hein, M.; Veith, U.; Jäger, V.; Peters, E.-M.; Peters, K.; von Schnering, H. G. : Einfache und variable Synthese optisch aktiver 1,2-Ami- noalkohole durch Grignard-Reaktion an Ν,Ο-Dibenzylglyceraldimin und -lactimin. Angew. Chem. 106 (1994) 1308-1311. Angew. Chem. Int. Ed.
Engl. 33 (1994) 1305-1308.
2. Franz, T.: Stereoselektive Synthesen von 3-Amino-l,2-<liolen, Isoxazo- lidinen und ß-Lactamen aus 2-O-Benzylglycerinaldehyd - SchlUsselstruk- turen zum Aufbau von Natur- und Wirkstoffen. Dissertation, Universität Wiiizburg, Germany 1992.
3. Krämer, B.; Franz, T.; Picasso, S.; Pnischek, P.; Jäger, V.: Glycono-1,3- lactams. Xylo Seríes: Stereoselective Access by [2+2] Cycloaddition, Exploratory Transformations, and Discovery of a New, Highly Selective Inhibitor of Glucoamylases. Synlett (1997) 295-297.
4. Henkel, S.; Krämer, Β.; Jäger, V.: Cf. structures of related ß-lactams. Ζ.
Kristallogr. NCS 212 (1997) 205-212.
5. Henkel, S.; Krämer, Β.; Jäger, V.: Crystal structure of (2Ä,l'/?)-2-hydroxy- 4-(r-phenylethyl)-3,4-dihydro-2H-l,4-oxazine-3-one, C12H13NO3. Ζ.
Kristallogr. NCS 212 (1997) 419-420.
6. Sheldrick, G. M.: Program Package SHELXTL-plus. Release 4.1.
Siemens Analytical X-Ray Instruments Inc., Madison (WI53719), US A 1990.