Z. Kristallogr. NCS 219 (2004) 53-54
© by Oldenbourg Wissenschaftsverlag, München
53
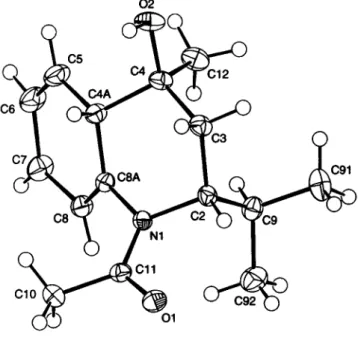
Crystal structure of (2ÄS,4S/?,4aSÄ)-l-acetyl-2-(l-methylethyl)-4-hydroxy- 4-methyl-l,2,3,4,4a,7-hexahydroquinoline, C15H22NO2
S. Gross
1,1. Briidgam", H. Hartl*
11and H.-U. Reißig
1I Freie Universität Berlin, Institut für Chemie - Organische Chemie, Takustr. 3, D-14195 Berlin, Germany
II Freie Universität Berlin, Institut für Chemie - Anorganische Chemie, Fabeckstr. 34-36, D-14195 Berlin, Germany Received December 18, 2003, accepted and available on-line February 5,2004; CCDC no. 1267/1192
Table 1. Data collection and handling.
Abstract
C 1 5 H 2 2 N O 2 ,
monoclinic,
P\2\!c\(no. 14),
a = 9.644(1) A, b = 8.750(1) A, c = 16.666(2) A, P = 103.733(2)°, V= 1366.1 A3, Z = 4,
Rgi(F) = 0.046, wRretfF2) = 0.146, T= 153 K.
Source of material
The title compound was obtained by samarium diiodide induced reaction of N-phenyl-AT-( 1 -isopropyl-3-oxo-1 -butyl)acetamide according to the procedure described in [ 1,2], purified by chroma- tography on silica gel and recrystallized from ethyl acetate; m.p.
437 K.
Discussion
The crystal structure proves the constitution and configuration of the title compound and also shows interesting details of the con- formation. The 4-methyl group and the bridgehead hydrogen at C4A and the isopropyl group at C2 occupy equatorial positions.
Bonding distances and bond angles show normal values.
Crystal:
Wavelength:
ft-
Diffractometer, scan mode:
20max:
N(hkl)rncasumi, N(hkl)unique:
Criterion for /obs, N(hkl)gc.
N(paramhcfmei'- Programs:
colorless prism, size 0.1 x 0.17 x 0.3 mm Mo Ka radiation (0.71073 A)
0.79 cm"1
Bruker SMART CCD, <plta with Acu = 0.3°
60.02°
16109,4000 /obs > 2 a(lobs), 3255 176
SHELXS-97 [3], SHELXL-97 [4], PLATON [5]
Table 2. Atomic coordinates and displacement parameters (in A2).
Atom Site X y z Uiso
H(2) 4e 0.1991 -0.0858 0.1334 0.022
H(3A) 4e 0.1780 -0.0166 0.0022 0.025
H(8B) 4e 0.3421 -0.0376 0.0374 0.025
H(5) 4e 0.2101 0.4796 -0.0491 0.034
H(6) 4e 0.2200 0.6724 0.0373 0.036
H(7) 4e 0.1736 0.6428 0.1743 0.034
H(8) 4e 0.1479 0.3775 0.2167 0.026
H(9) 4e 0.4107 0.1370 0.1963 0.027
H(91B) 4e 0.5600 -0.0780 0.2430 0.054 H(91C) 4e 0.5176 -0.0662 0.1464 0.054 H(91A) 4e 0.4405 -0.1844 0.1916 0.054 H(92B) 4e 0.2945 -0.0774 0.2892 0.050
H(92A) 4e 0.2569 0.0971 0.2851 0.050
H(92C) 4e 0.4123 0.0431 0.3268 0.050
H(10A) 4e -0.2002 0.1422 0.1520 0.038 H(10B) 4e -0.1195 0.2653 0.1120 0.038 H(10C) 4e -0.0858 0.2477 0.2083 0.038
H(12B) 4e 0.5061 0.1681 0.0484 0.042
H(12A) 4e 0.4419 0.2849 0.1011 0.042
H(12C) 4e 0.4582 0.3307 0.0129 0.042
H(21) 4e 0.195(3) 0.144(3) -0.104(1) 0.059(6) H(4A) 4e 0.082(2) 0.257(2) -0.015(1) 0.033(4)
* Correspondence author (e-mail: hartl@chemie.fu-berlin.de)
54 C15H22NO2
Table 3. Atomic coordinates and displacement parameters (in Â2).
Atom Site X y z I/11 V22 UÌÌ Un Un U2)
CKD 4e -0.0145(1) -0.0585(1) 0.16713(6) 0.0268(4) 0.0222(4) 0.0287(4) -0.0025(3) 0.0096(3) 0.0045(3) 0(2) 4e 0.2800(1) 0.1784(1) -0.07809(5) 0.0359(5) 0.0336(5) 0.0202(4) -0.0070(4) 0.0147(4) -0.0044(3) N(l) 4e 0.1254(1) 0.1274( 1 ) 0.13395(6) 0.0203(4) 0.0153(4) 0.0196(4) 0.0019(3) 0.0075(3) 0.0009(3) C(2) 4e 0.2379(1) 0.0161(1) 0.12788(7) 0.0195(5) 0.0145(5) 0.0215(5) 0.0014(4) 0.0064(4) -0.0007(4) C(3) 4e 0.2612(1) 0.0264(1) 0.04000(7) 0.0250(5) 0.0187(5) 0.0214(5) 0.0003(4) 0.0090(4) -0.0039(4) C(4) 4e 0.2876(1) 0.1867(1) 0.00853(7) 0.0250(5) 0.0212(5) 0.0193(5) -0.0019(4) 0.0099(4) -0.0026(4) C(4A) 4e 0.1698(1) 0.2964(1) 0.02417(7) 0.0255(5) 0.0175(5) 0.0181(5) -0.0001(4) 0.0070(4) 0.0007(4) C(5) 4e 0.1961(2) 0.4591(1) 0.00321(8) 0.0404(7) 0.0213(5) 0.0259(6) 0.0001(5) 0.0131(5) 0.0060(4) C(6) 4e 0.2006(2) 0.5756(1) 0.05492(9) 0.0407(7) 0.0180(5) 0.0328(6) -0.0009(5) 0.0117(5) 0.0046(5) C(7) 4e 0.1762(2) 0.5601(1) 0.13968(8) 0.0418(7) 0.0163(5) 0.0276(6) -0.0002(5) 0.0096(5) -0.0025(4) C(8) 4e 0.1565(1) 0.3979(1) 0.16329(7) 0.0260(5) 0.0183(5) 0.0211(5) 0.0008(4) 0.0072(4) -0.0003(4) C(8A) 4e 0.1508(1) 0.2817(1) 0.11119(7) 0.0200(5) 0.0160(5) 0.0190(5) 0.0015(4) 0.0063(4) 0.0018(4) C(9) 4e 0.3700(1) 0.0355(1) 0.20027(7) 0.0223(5) 0.0190(5) 0.0239(5) -0.0002(4) 0.0034(4) 0.0010(4) C(91) 4e 0.4825(2) -0.0844(2) 0.1948(1) 0.0257(6) 0.0372(7) 0.0421(8) 0.0094(5) 0.0025(5) 0.0002(6) C(92) 4e 0.3297(2) 0.0235(2) 0.28299(8) 0.0335(7) 0.0422(8) 0.0218(6) 0.0013(6) 0.0030(5) 0.0032(5) C(10) 4e -0.1108(1) 0.1939(2) 0.15662(8) 0.0227(5) 0.0272(6) 0.0276(6) 0.0034(4) 0.0097(4) 0.0011(4) COD 4e 0.0037(1) 0.0787(1) 0.15293(7) 0.0214(5) 0.0215(5) 0.0157(4) -0.0002(4) 0.0054(4) 0.0009(4) C(12) 4e 0.4374(1) 0.2483(2) 0.04625(8) 0.0247(6) 0.0304(6) 0.0313(6) -0.0065(5) 0.0108(5) -0.0028(5)
Acknowledgments. Support of this work by the Schering Research Founda- tion, the Volkswagen-Stiftung and the Fonds der Chemischen Industrie is gratefully acknowledged.
References
1. Gross, S.; Reissig, H.-U.: Samarium Diiodide-Induced Diastereo- selective Synthesis of Hexahydroquinoline Derivatives. Synlett (2002) 2027-2030.
2. Gross, S.: Samariumdiiodid-induzierte Cyclisierungen zu JV-Hetero- cyclen - Neue Möglichkeiten fur die Alkaloidsynthese. Dissertation, Freie Universität Berlin (2003).
3. Sheldrick, G. M.: Phase Annealing in SHELX-90: Direct Methods for Larger Structures. Acta Ciystallogr. A46 (1990) 467-473.
4. Sheldrick, G. M.: SHELXL-97. A Program for the Refinement of Crystal Structures. University of Gôttingen, Germany 1997.
5. Spek, A. L.: PLATON - A Multipurpose Ciystallographic Tool. Utrecht University, The Netherlands 1998.