720 Ζ. Kristallogr. NCS 213 (1998) 720-722
© by R. Oldenbourg Verlag, München
Crystal structures of the diastereomeric 2-bromo-3-methyl-l-(2-methyl- phenyl)-l-phenyl-l-butenes, С4Н4(СбН5)(С7Н7)ВгСНз
к . Peters, E.-M. Peters
Max-Planck-Institut fiir Festkörperforschung, Heisenbergstraße 1, D-70506 Stuttgan, Germany
U. M. Doht and H. Quast
Universität WUrzburg, Institut fiir Organische Chemie, Am Hubland, D-97074 Würzburg, Germany
Received April 27, 1998, CSD-No. 409300 and CSD-No. 409301
Source of material: A mixture of the diastereomeric title com- pounds ( £ : Z = 18:82) was prepared, according to ref. 1, by bromination of 3-methyl-l-(2-methylphenyl)-l-phenyl-1-butene (E:Z = 1:3, carbon tetrachloride solution, 273 К - 313 K, Ih) with one equivalent of bromine followed by distillation of the oily product at 423 К - 433 К (bath temperature) / 0.01 Torr. Sepa- ration of the diastereomers was achieved by recrystallization from methanol. Crystallization of the distilled product yielded the Ζ diastereomer which was recrystallized fíve times to afford color- less crystals, mp 339 К - 340 К, in 70% yield. Addition of a seed crystal of the E diastereomer of the corresponding chloro com- pound, see ref. 2, to the mother liquors of the Ζ diastereomer induced crystallization of the E diastereomer. Ten recrystallizations from methanol afforded colorless crystals, mp 316 К - 317 К in 3%
yield).
The crystal structures of the title compounds provide unequivocal proof of their confígurations which is more difñcult to obtain on the basis of spectroscopic evidence. Both diastereomers are chiral by virtue of the presence of a helical axis. The stereoprahic projection- sexhibit the enantiomers with M,E (Figure 1) and P, Ζ configuration (Figure 2). In both diastereomers the isopropyl group at C2 adopts a conformation in which the H-C3 bond lies in the plane of the double bond.
C8 C7
Pig. 1. Molecule plot of the P, Ζ diastereomer of С4Н4(СбН5)(С7Н7)ВгСНз.
Ci8Hi9Br, orthorhombic, Pcali (No. 29), a =27.643(6) Â,
¿>=6.333(1) Â, с =8.850(2) Â, V=1549.3Â^ Z=4, =0.043, Ry^F) =0.036.
Table 1. Parameters used for the X-ray data collection
As shown by the Newman projections (above) the planes of the aromatic rings are twisted strongly relative to the plane of the double bond. The planes of the o-tolyl rings are displaced farther from the plane of the double bonds ( £ diastereomer: 79.4°, Ζ diastereomer: 88.4°) than those of the phenyl rings ( £ diastere- omer: 66.3°, Ζ diastereomer: 65.1°). The crystal structures of the corresponding diastereomeric 2-chloro compounds are reported in ref. 2.
Crystal: colorless prism, size 0.35 χ 0.45 0.85 mm Wavelength: Mo Ka radiation (0.71073 Â)
μ: 26.40 cm-'
Diñractometer: SYNTEXP3
Scan mode: Wyckoff
293 К
2вта,: 55°
1897 Criterion for Fo. Ро>Ъа(Ро)
^(param)rtfine¡r· 172
Program: SHELXTL-plus
Diastereomers С4Н4(СбН5)(С7Н7)ВгСНз 7 2 1
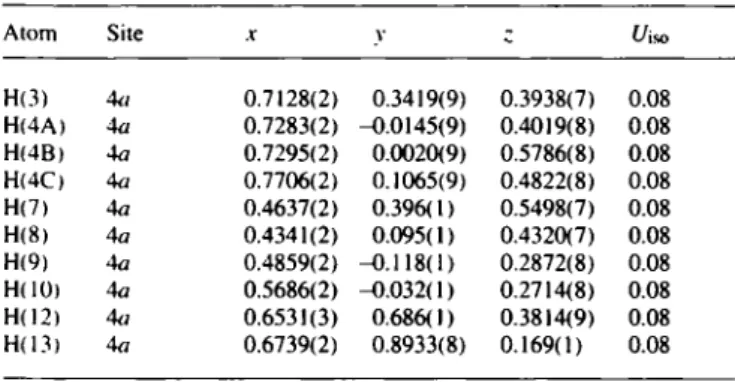
Table 2. Final atomic coordinates and displacement parameters (in Â^) Table 2. (Continued)
Atom Site X V ; t/iso Atom Site X V с и,so
H(3) A(t Qima) 0.3419(9) 0.3938(7) 0.08 H ( I 4 ) 4 a 0.6659(3) 0.760(1) - 0 . 0 7 3 ( 1 ) 0.08
H(4A) 4 a 0.7283(2) - 0 . 0 1 4 5 ( 9 ) 0.4019(8) 0.08 H(15) 4 a 0.6325(3) 0.425(2) - 0 . 1 0 9 3 ( 9 ) 0.08
H(4B) 4 a 0.7295(2) 0.0020(9) 0.5786(8) 0.08 H ( I 6 ) 4 a 0.6121(2) 0.212(1) 0.0955(7) 0.08
H(4C) 4 a 0.7706(2) 0.1065(9) 0.4822(8) 0.08 H ( I 7 A ) 4a 0.7522(2) 0.448(1) 0.6151(8) 0.08
H(7) 4 a 0.4637(2) 0.396(1) 0.5498(7) 0.08 H(17B) 4a 0.7106(2) 0.346(1) 0.7107(8) 0.08
H(8) 4 a 0.4341(2) 0.095(1) 0.4320(7) 0.08 H(17C) 4a 0.7001(2) 0.546(1) 0.6116(8) 0.08
H(9) 4 a 0.4859(2) - 0 . 1 1 8 ( 1 ) 0.2872(8) 0.08 H ( 1 8 A ) 4a 0.5871(2) 0.563(1) 0.5605(8) 0.08 H(IO) 4 a 0.5686(2) - 0 . 0 3 2 ( 1 ) 0.2714(8) 0.08 H ( I 8 B ) 4a 0.5457(2) 0.532(1) 0.6793(8) 0.08
H ( I 2 ) 4a 0.6531(3) 0.686(1) 0.3814(9) 0.08 H(18C) 4 a 0.5371(2) 0.675(1) 0.5369(8) 0.08
H ( I 3 ) Aa 0.6739(2) 0.8933(8) 0.169(1) 0.08
Table 5. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X >· г Vu t/22 t/зз Un Un ί/23
Br 4a 0.63126(2) 0.04605(9) 0.6533 0.0566(3) 00698(4) 00584(3) -0.0114(3) -0.0015(6) 0.0210(5) C(l) 4a 0.6178(2) 0.2874(8) 0.3919(7) 0.043(3) 0042(3) 0049(3) -0.003(2) αοο4(3) -0.003(3) C(2) 4a 0.6511(2) 0.2204(8) 0.4865(6) 0.041(3) 0.047(3) OW8(3) -0.004(2) -0.003(3) 0.008(3) C(3) 4a 0.7052(2) 0.2704(9) 0.4865(7) 0.037(3) 0.061(4) 0.058(4) -0.006(3) -0.004(3) 0.015(3) C(4) 4a 0.7367(2) 0.0742(9) 0.4859(8) 0.046(3) 0.096(5) 0.097(6) 0.010(3) 0.003(4) 0.024(5) C(5) 4a 0.5652(2) 0.2317(8) 0.4044(6) 0.037(3) 0.053(3) 0.041(3) 0.001(2) -0.002(2) 0.005(3) C(6) 4a 0.5344(2) 0.3588(9) 0.4901(7) 0.051(3) 0.050(3) 0.053(4) 0.001(3) -0.001(3) 0.000(3) C(7) 4a 0.4854(2) 0.304(1) 0.4967(7) 0.040(3) 0081(5) 0.057(4) 0.017(3) 0.005(3) -0.001(4) C(8) 4a 0.4678(2) 0.129(1) 0.4242(7) 0.038(3) 0093(5) 0.060(4) -0.005(3) -0.002(3) 0.005(4) C(9) 4a 0.4988(2) 0.003(1) 0.3377(8) 0.050(3) 0078(4) 0.080(4) -0.014(3) -0.006(4) -0.014(4) C(10) 4a 0.5473(2) 0.056(1) 0.3287(8) 0.047(3) 0059(4) 0.066(4) -0.001(3) 0.003(3) -0.005(4) C(ll) 4a 0.6310(2) 0.427(1) 0.2594(8) 0.038(3) 0.053(5) 0049(5) 0.006(3) 0.002(3) 0012(3) C(I2) 4a 0.6489(3) 0.631(1) 0.2811(9) 0.046(3) 0.056(4) 0.061(5) -0.008(4) -0.002(3) 0.000(4) C(I3) 4a 0.6616(2) 0.7526(8) 0.157(1) αο56(3) 0.062(3) 0.074(4) 0.009(3) 0.009(5) 0.027(6) C(I4) 4a 0.6567(3) 0.677(1) 0.013(1) α047(4) 0.083(6) 0.070(5) 0.012(4) 0.008(4) 0.030(5) C(15) 4a 0.6374(3) 0.476(2) -0.0083(9) 0.057(4) 0.117(8) 0.044(4) 0.018(5) 0.002(4) 0.010(5) C(16) 4a 0.6250(2) 0.351(1) 0.1118(7) 0.051(4) 0063(4) 0.056(5) 0.006(3) -0.006(3) αοο5(3) C(I7) 4a 0.7183(2) 0.417(1) 0.6179(8) 0.060(3) 0091(5) 0.092(7) -0.030(3) -0.016(4) 0.007(4) C(I8) 4a 0.5528(2) 0.550(1) 0.5739(8) 0079(4) 0.068(4) 0.075(4) 0.011(4) 0.015(4) -0.010(4)
2. Л/, £ D i a s t e r e o m e r
C9
Ci8Hi9Br, monocUnic, Plik (No. 14), a =12.544(3) Â, Ъ =9.885(2) Â, с =12.888(3) Â, β =97.79(3)°, V=1583.3 Л ' , Ζ =4, R(F) =0.060, Ry^F) =0.052.
Table 4. Parameters used for the X-ray data collection
Crystal: colorless prism, size 0.65 χ 0.85 χ 0.35 nun Wavelength: Mo Ka radiation (071073 Â)
μ: 25.80 cm-'
Diffractometer: SYNTEXP3
Scan mode: Wyckoff
'^measurement· 293 К
28max: 55°
2495 Criterion for Fo- F o > 3 a ( f o )
^{param)refineir. 173
Program: SHELXTL-plus
Fig. 2. Molecule plot of the M, E diastereomer of С4Н4(СбН5)(С7Н7)ВгСНз.
722
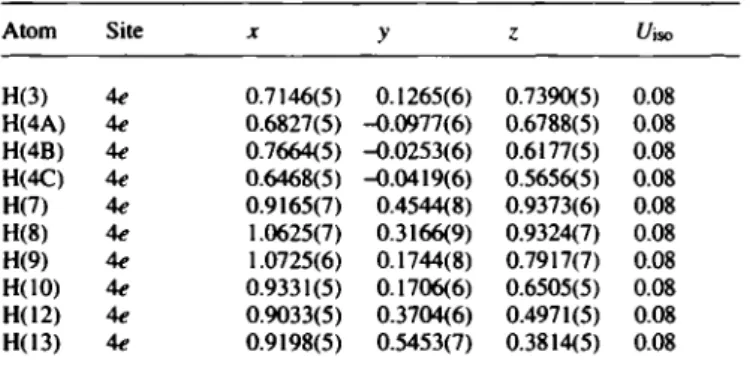
Diastereomers С4Н4(СбН5)(С7Н7)В|СНзTable 5. Final atomic coordinates and displacement parameters (in Â^) Table 5. (Continued)
Atom Site X У ζ ί/iso Atom Site X У Ci«,
H(3) 4e 0.7146(5) 0.1265(6) 0.7390(5) 0.08 Н(14) 4е 0.7924(6) 0.7145(7) 0.3553(5) 0.08 H(4A) 4e 0.6827(5) -Ц).0977(6) 0.6788(5) 0.08 Н(15) 4е 0.6451(6) 0.7108(7) 0.4467(6) 0.08 H(4B) 4€ 0.7664(5) -0.0253(6) 0.6177(5) 0.08 Н(16) 4е 0.6247(5) 0.5336(7) 0.5623(5) 0.08 H(4C) 4e 0.6468(5) -0.0419(6) 0.5656(5) 0.08 Н(17А) 4е 0.5472(5) 0.0311(7) 0.7555(5) 0.08 H(7) 4e 0.9165(7) 0.4544(8) 0.9373(6) 0.08 Н(17В) 4е 0.5010(5) 0.0957(7) 0.6476(5) 0.08 H(8) 1.0625(7) 0.3166(9) 0.9324(7) 0.08 Н(17С) 4е 0.5390(5) 0.1888(7) 0.7440(5) 0.08 H(9) 4e 1.0725(6) 0.1744(8) 0.7917(7) 0.08 Н(18А) 4е 0.6933(6) 0.4774(7) 0.7404(5) 0.08 H(10) 4€ 0.9331(5) 0.1706(6) 0.6505(5) 0.08 Н(18В) 4е 0.7672(6) 0.5793(7) 0.8103(5) 0.08 H(I2) 4e 0.9033(5) 0.3704(6) 0.4971(5) 0.08 Н(18С) 4е 0.7105(6) 0.4610(7) 0.8625(5) 0.08 H(I3) 4e 0.9198(5) 0.5453(7) 0.3814(5) 0.08
Table 6. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X У г í / l l t / 2 2 t / з з Un t / | 3 í / 2 3
Br Ae 0.57933(5) 0.22317(7) 0.47945(5) 0.0772(5) 0.1142(6) 0.0887(5) -0.0116(4) -0.0134(3) -0.0063(5) C d ) 4« 0.7532(4) 0.3206(5) 0.6180(4) 0.049(3) 0.058(4) 0.067(4) 0.004(3) 0.009(3) -0.009(3) C(2) Ле 0.6792(4) 0.2252(6) 0.6049(4) 0.054(3) 0.072(4) 0.065(4) 0.004(3) 0.006(3) -0.007(3) C(3) 4« 0.6651(5) 0.1106(6) 0.6766(5) 0.067(4) 0.077(5) 0.078(4) -0.020(3) 0.000(3) -0.012(4) C(4) 4e 0.6927(5) -0.0265(6) 0.6304(5) 0.108(6) 0.082(5) 0.118(6) -0.020(4) -0.004(4) 0.009(5) C(5) Ле 0.8404(4) 0.3166(6) 0.7101(4) 0.061(4) 0.064(4) 0.068(4) -0.016(3) 0.012(3) -0.006(3) C(6) 4€ 0.8364(5) 0.3986(6) 0.7949(5) 0.077(4) 0.063(4) 0.097(5) -0.022(4) 0.016(4) 0.005(4) C(7) 4« 0.9196(7) 0.3968(8) 0.8778(6) 0.130(7) 0.118(7) 0.076(5) -0.059(6) -0.017(5) 0.004(5) C(8) 4« 1.0051(7) 0.3161(9) 0.8750(7) 0.094(6) 0.146(9) 0.106(7) -0.059(6) -0.035(6) 0.049(6) C(9) 4e 1.0112(6) 0.2324(8) 0.7919(7) 0.059(4) 0.135(7) 0.139(7) -0.010(5) 0.002(5) 0.048(6) C(IO) Ле 0.9285(5) 0.2300(6) 0.7086(5) 0.055(4) 0.107(5) 0.098(5) -0.001(4) 0.007(3) 0.021(4) C ( l l ) 4e 0.7624(4) 0.4329(6) 0.5429(4) 0.048(3) 0.067(4) 0.075(4) 0.004(3) 0.003(3) -Ч).011(3) C(12) 4c 0.8492(5) 0.4396(6) 0.4873(5) 0.069(4) 0.062(4) 0.093(5) 0.005(3) 0.013(4) 0.003(4) C(13) 4c 0.8588(5) 0.5427(7) 0.4190(5) 0.072(4) 0.085(5) 0.107(5) 0.000(4) 0.026(4) -0.001(5) C(14) 4e 0.7846(6) 0.6428(7) 0.4039(5) 0.112(6) 0.066(5) 0.097(5) -0.004(4) 0.006(5) 0.011(4) C(15) 4c 0.6978(6) 0.6399(7) 0.4570(6) 0.092(6) 0.084(5) 0.127(7) 0.034(4) 0.008(5) 0.013(5) C(16) 4c 0.6869(5) 0.5364(7) 0.5264(5) 0.075(4) 0.089(5) 0.107(5) 0.025(4) 0.030(4) 0.012(4) C(I7) 4c 0.5528(5) 0.1061(7) 0.7091(5) 0.101(6) 0.122(6) 0.088(5) -0.029(5) 0.012(4) 0.001(4) C d 8) 4c 0.7440(6) 0.4868(7) 0.8028(5) 0.160(8) 0.091(5) 0.092(5) -0.021(5) 0.035(5) -0.026(4)
References
1. Doht, U. M. : 1,2-Disubstituierte 2-Halogenstyrole. - Synthese und Stereo- chemie der 1,2-Eliminierung zu Alienen. Dissertation, Universität WUrz- burg, Germany 1986.
2. Peters, K.; Peters, E.-M.; Doht, U. M.; Quast, H.: Crystal structures of the diastereomeric 24;hloro-3-methyl-1 -(2-methylphenyl)-1 -phenyl-1 -bute- nes, С4Н4(СбН5)(С7Н7)СНСНз. Ζ. Kristallogr. NCS 213 (1998) 717- 719.
3. Sheldrick, G. M.: Program Package SHELXTL-plus. Release 4.1.
Siemens Analytical X-Ray Instruments Inc., Madison (Wl 53719), USA 1990.