ζ. Kristallogr. NCS 213 (1998) 795-796
795
© by R. Oldenbourg Verlag, München
Crystal structure of methyl iV,0-diacetyl-a-L-acosaminide, C11H19NO5
S. Henkel, A. Menzel and V. Jäger
Universität Stuttgart. Institut für Organische Chemie, Pfaffenwaldring 55. D-70569 Stuttgart, Germany Received April 15, 1998, CSD-No. 409292
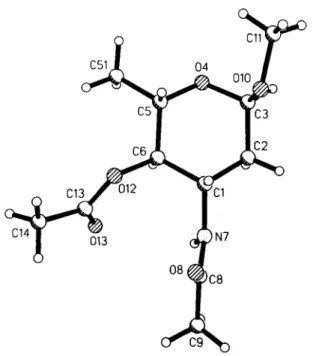
Source of material: The title compound is a derivative of L-aco- samine, which forms part of highly active cancerostatics such as epirubicin (see ref. 1 ), and which has repeatedly been synthesized before (see refs. 2-3). In a new synthesis the title compound (see refs. 4-6) was prepared by Henry reaction (see refs. 4-8) of a lactaldehyde derivative with nitropropanal dimethylacetal (see refs. 9) followed by cyclization to the methyl glycoside. This was reduced and acetylated to get the title compound, (mp 432 K;
- 1 9 1 , c = 0.54,MeOH).
The molecules are stacked in four columns, in each one with identical orientation, linked by an intermolecular hydrogen bridge of the amido group (N7) to the oxygen atom (08) of the N-acetyl group. The molecules adopt a 4C conformation with the 1-methoxy group oriented axially and all other ring substituents placed equa- tonally.
Table 3. Final atomic coordinates and displacement parameters (in Â^)
CiiHi9N05,orthorhombic,/^l2i2i (No. 19), α =5.021(1) À,
¿=12.857(2) Â, с =20.978(4) Â, V=1354.2Â^ Z=4, R(F) =0.052, Rv^F^) =0.124.
Table 1. Parameters used for the X-ray data collection
Ciystal: colorless block, size 0.3 χ 0.5 χ 0.7 mm Wavelength: Mo Ka radiation (0.71073 Â)
μ: 0.95 cm"'
Diffractometer: NicoletPS
Scan mode: Wyckoff
Tmeasur^n,: 293 К
2 в т « : 51.96°
N(AW)^«,: 1482
Criterion for ¡0. /ο>2σ(/ο)
^(рагатУгфтГ· 155
Programs: SHELXS-86, SHELXL-93
Table 2. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X У г í/iso
H(l) 4a 0.8835(8) 1.1028(3) 0.5713(2) 0.065
H{2A) 4a 0.5855(8) 1.2039(3) 0.5143(2) 0.078 H(2B) 4a 0.3419(8) 1.1520(3) 0.5486(2) 0.078 H(3) 4a 0.403(1) 1.3113(3) 0.5937(2) 0.087 H(5) 4a 0.8193(9) 1.1587(3) 0.6883(2) 0.077 H(51A) 4a 0.550(1) 1.1592(4) 0.7797(2) 0.144
H(51B) 4a 0.648(1) 1.0454(4) 0.7668(2) 0.144 H(51C) 4a 0.357(1) 1.0780(4) 0.7484(2) 0.144 H(6) 4a 0.4438(8) 1.0144(3) 0.6375(2) 0.067
H(7) 4a 0.5036 0.9600 0.5243 0.075
H(9A) 4a 0.591(1) 0.8588(4) 0.4498(2) 0.141 H(9B) 4a 0.854(1) 0.7988(4) 0.4662(2) 0.141 H(9C) 4a 0.849(1) 0.8785(4) 0.4097(2) 0.141 H(llA) 4a 0.986(2) 1.4220(3) 0.6375(3) 0.167 H ( l l B ) 4a 0.762(2) 1.3825(3) 0.6837(3) 0.167
H(llC) 4a 0.686(2) 1.4466(3) 0.6227(3) 0.167 H(14A) 4a 1.099(1) 0.8458(4) 0.7025(3) 0.166 H(14B) 4a 1.027(1) 0.7564(4) 0.6548(3) 0.166 H(14C) 4a 0.884(1) 0.7628(4) 0.7212(3) 0.166
Atom Site X У ζ Un U22 ί/33 Un t/l3 ί/23
C d ) 4a 0.6965(8) 1.0810(3) 0.5711(2) 0.040(2) 0.063(2) 0.060(2) -0.005(2) 0.004(2) -0.010(2) C(2) 4a 0.5245(8) 1.1747(3) 0.5544(2) 0.051(2) 0.080(3) 0.064(2) -0.003(2) -0.002(2) 0.005(2) C(3) 4a 0.533(1) 1.2574(3) 0.6048(2) 0.073(3) 0.069(2) 0.074(3) 0.019(2) 0.007(3) 0.009(2) 0 ( 4 ) 4a 0.4660(6) 1.2168(2) 0.6655(1) 0.079(2) 0.076(2) 0.071(2) 0.017(2) 0.022(2) 0.002(2) C(5) 4a 0.6356(9) 1.1334(3) 0.6853(2) 0.068(2) 0.067(2) 0.057(2) 0.000(2) 0.004(2) 0.002(2) C(51) 4a 0.539(1) 1.1010(4) 0.7511(2) 0.133(5) 0.092(3) 0.063(2) 0.008(4) 0.018(3) 0.003(2) C(6) 4a 0.6230(8) 1.0447(3) 0.6378(2) 0.045(2) 0.063(2) 0.059(2) -0.003(2) 0.002(2) -0.004(2) N(7) 4a 0.6633(6) 1.0000(3) 0.5241(2) 0.040(2) 0.079(2) 0.070(2) -0.007(2) -0.001(2) -0.017(2)
796
Methyl yV,0-diacetyl-a-L-acosaminideTable 3. (Continued)
Atom Site X У ζ t/ll ί/22 ί/зз 1/12 U\ì Uli
C(8) 4a 0.8600(8) 0.9489(3) 0.4963(2) 0.050(2) 0.081(3) 0.065(2) 0.000(2) -0.001(2) -0.013(2) СЧ8) 4a 1.0961(6) 0.9704(3) 0.5066(2) 0.041(2) 0.138(3) 0.130(3) -0.001(2) 0.006(2) -0.054(3) C(9) 4c 0.781(1) 0.8636(4) 0.4515(2) 0.083(3) 0.102(3) 0.097(3) 0.006(3) -0.002(3) -0.038(3) O(I0) 4a 0.7870(7) 1.3017(2) 0.6042(1) 0.087(2) 0.061(2) 0.081(2) -0.007(2) 0.012(2) -0.005(2) C ( l l ) 4a 0.807(2) 1.3959(3) 0.6399(3) 0.149(6) 0.071(3) 0.115(4) -41.011(4) 0.008(4) -0.017(3) 0(12) 4a 0.8137(6) 0.9684(2) 0.6597(1) 0.058(2) 0.058(2) 0.077(2) -0.001(2) -0.006(2) -0.000(1) C(13) 4a 0.750(1) 0.8681(4) 0.6588(3) 0.066(3) 0.068(3) 0.105(4) -0.002(3) 0.012(3) 0.006(3) 0(13) 4a 0.5481(9) 0.8363(3) 0.6352(2) 0.099(3) 0.085(2) 0.180(4) -0.024(2) -0.022(3) 0.003(3) C(14) 4a 0.958(1) 0.8026(4) 0.6867(3) 0.094(4) 0.082(3) 0.155(5) 0.020(3) 0.004(4) 0.019(4)
Acknowledgments. We are grateful to the Fonds der Chemischen Industrie for fínancial support and to Dr. Wolfgang Frey for help with the preparation of the files.
References
1. Foye, W. O. ACS Professional Reference Book, Washington DC 1995.
2. Hauser, F. M.; EUenberger, S. R.: Synthesis of 2,3,6-Trideoxy-3-amino- and 2,3,6-Trideoxy-3-mtrohexoses. Chem. Rev. 86 (1986) 35-67.
3. Socha, D.; Juiczak, M.; Chmielewski, M.: Synthesis of Acosamine and Daunosamine пхип Sugar δ-Enelactones. Tetrahedron S3 (1997) 739-746.
4. Jäger, v.; Menzel, Α.: Unpublished results. Universität Stuttgart 1995.
5. Menzel, Α.: Dissertation, Universität Stuttgart, in preparation.
6. Öhrlein, R.: Darstellung von natürlichen und unnatürlichen 3-Ainino- und 3-Nitrohexose-Derivaten. Dissertation, Universtität Würzburg, Germany
1988.
7. Henry, L.: Formation synthétique d'alcools nitrés. С. R. hebd. Séances Acad. Sci. 120(1895) 1265-1268.
8. Jäger, v.; Wehner, V.: Synthese von D- und L-2-Aimno-2-desoxyarabino- se sowie von D- und L-l,4-Didesoxy-l,4-iniinolyxit durch (C2+C3)-Ni- troaldol-Addition mit 2-O-BenzylgIycerinaldehyd, Angew. Chem. 102 (1990) 1180-1182; Angew. Chem. Int. Ed. Engl. 29 (1990) 1169-1171.
9. Griesser, H.; Öhrlein, R.; Schwab, W.; Ehrler, R.; Jäger, V.: 3-Nitropro- panal, 3-Nitropropanol and 3-Nitropropanal Dimethyl Acetal. Org. Synth., accepted for checking.
10. Sheldrick, G. M.: Phase Annealing in SHELX-90: Direct Methods for Large Structures. Acta Crystallogr. A46 (1990) 467^73.
11. Sheldrick, G. M.: SHELXL-93. Program for refining crystal structures.
University of Göttingen, Germany 1993.