ζ. Kristallogr. NCS 213 (1998) 717-719
717
© by R. Oldenbourg Verlag, München
Crystal structures of the diastereomeric 2-chloro-3-methyl-l-(2-methyl- phenyl)-l-phenyl-l-butenes, С4Н4(СбН5)(С7Н7)С1СНз
к . Peters, E.-M. Peters
Max-Planck-Institut für Festkörperforschung, Heisenbergstraße I, D-70506 Stuttgart, Germany U. M. Doht and H. Quast
Universität Würzburg, Institut für Organische Chemie, Arn Hubland, D-97074 Würzburg, Germany
Received April 27, 1998, CSD-No. 409298 and CSD-No. 409299
Source of material: Mixtures of equal amounts of the diastere- omeric title compounds were prepared, according to ref. 1, by chlorination of 3-methyl-l-(2-methylphenyl)-l-phenyl-1-butene (£:Z = 1:3, carbon tetrachloride solution, 268 К - 278 К) with an excess of chlorine followed by thermal dehydrochlorination at 423 К - 443 К of the colorless intermediate 1,2-dichloride {0.5h - Ih) and distillation at 0.1 Torr. Partial separation of the dias- tereomers was achieved by careful distillation of the 1 : 1 mixture through a 60 cm Spaltrohr colurrm (bath temp. 4 1 8 K 1 2 K / 0 . 1 Torr) which afforded fractions with maximal 66% of the Ζ dias- tereomer (bp 377 К - 378 К / 0.1 Torr) and maximal 98% of the
E diastereomer (bp >378 К / 0.1 Torr). Five to ten recrystalliza-tions of the enriched fractions eventually furnished the pure diastereomers as colorless crystals, each in 27% yield, mp of the
E diastereomer 325 К - 326 К, of the Ζ diastereomer 319 К -320 К.
The crystal structures of the title compounds provide unequivocal proof of their configurations which is more difficult to obtain on the basis of spectroscopic evidence. Both diastereomers are chiral by virtue of the presence of a helical axis. The stereographic projections exhibite the enatiomers with M, E (Figure 2) and P, Ζ configuration (Figure 1 ). In both diastereomers the isopropyl group at C2 adopts a conformation in which the H-C3 bond lies in the plane of the double bond.
65-1
As shown by the Newman projections (above), the planes of the aromatic rings are twisted strongly relative to the plane of the double bond. This twisting is more pronounced for the Ζ diastereomer than for the E diastereomer. Furthermore, the planes of the o-tolyl rings are displaced farther from the plane of the double bonds (£ diaste- reomer: 78.7°, Ζ diastereomer: 88.1°) than those of the phenyl rings
(E diastereomer: 63.1 Ζ diastereomer 65.0°). The crystal structuresof the corresponding diastereomeric 2-bromo compounds are repor- ted in ref 2.
1. Ζ Diastereomer
C14
Fig. 1. Molecule plot of the P, Ζ diastereomer of С4Н4(СбН5)(С7Н7)С1СНз.
CisHigCl, orthoriiombic, Pca2\ (No. 29), a =27.636(6) Â,
¿>=6.272(1) Â, с =8.809(2) Â, V=1526.9Â^ Z=4, =0.045, ÄwfFJ =0.043.
Table 1. Parameters used for the X-ray data collection
Crystal: colorless prism, size 0.45 χ 0.45 χ 0.65 mm Wavelength: Mo Ka radiation (0.71073 Â)
μ: 2.30 cm"'
Difiractometer: SYNTEXP3
Scan mode: Wyckoff
'^measunmetu'· 293 К 55°
ЩЬк1)т1чие·· 1341 Criterion for Fo: F o > 3 a ( F „ ) iHparam)reimeir. 171
Program: S^ÍF,I,XTL·plus
718
Diastereomers С4Н4(СбН5)(С7Н7)С1СНзTable 2. Final atomic coordinates and displacement parameters (in Â^) Table 2. (Continued)
Atom Site X У г í/iso Atom Site X V ; t/iso
H(3) Aa 0.7113(1) 0.3264(7) 0.4065(6) 0.08 Н(14) 4а 0.6655(2) 0.745(1) -0.0634(7) 0.08 H(4A) Λα 0.7263(2) -0.0357(8) 0.4172(8) 0.08 Н(15) 4а 0.6324(2) 0.409(1) -0.1014(7) 0.08 H(4B) 4a 0.7275(2) -0.0189(8) 0.5947(8) 0.08 Н(16) 4а 0.6115(1) 0.1944(8) 0.1073(6) 0.08 H(4C) Aa 0.7685(2) 0.0873(8) 0.4977(8) 0.08 Н(17А) 4а 0.7514(2) 0.4318(8) 0.6286(8) 0.08 H(7) 4a 0.4627(1) 0.3968(8) 0.5596(6) 0.08 Н(17В) 4а 0.7098(2) 0.3287(8) 0.7246(8) 0.08 H(8) 4a 0.4327(2) 0.0977(9) 0.4404(6) 0.08 Н(17С) 4а 0.6994(2) 0.5310(8) 0.6253(8) 0.08 H(9) 4a 0.4838(2) -0.124(1) 0.2996(7) 0.08 Н(18А) 4а 0.5868(2) 0.5572(8) 0.5717(7) 0.08 H(IO) 4a 0.5670(2) -0.0438(8) 0.2859(6) 0.08 Н(18В) 4а 0.5453(2) 0.5264(8) 0.6911(7) 0.08 H(12) 4a 0.6531(2) 0.6739(8) 0.3913(6) 0.08 Н(18С) 4а 0.5368(2) 0.6705(8) 0.5482(7) 0.08 H(13) 4a 0.6738(2) 0.8814(8) 0.1791(7) 0.08
Table 3. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X y ζ í/ll U22 ί/зз Ui2 Uli {/23
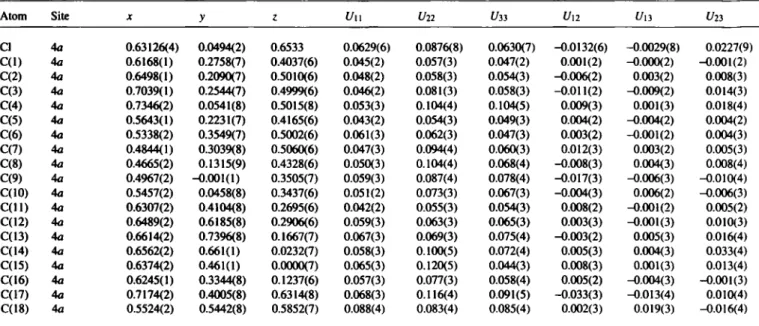
Cl 4a 0.63126(4) 0.0494(2) 0.6533 0.0629(6) 0.0876(8) 0.0630(7) -0.0132(6) -0.0029(8) 0.0227(9) C(l) 4a 0.6168(1) 0.2758(7) 0.4037(6) 0.045(2) 0.057(3) 0.047(2) 0.001(2) -0.000(2) -0.001(2) C(2) 4a 0.6498(1) 0.2090(7) 0.5010(6) 0.048(2) 0.058(3) 0.054(3) -0.006(2) 0.003(2) 0.008(3) C(3) 4a 0.7039(1) 0.2544(7) 0.4999(6) 0.046(2) 0.081(3) 0.058(3) -0.011(2) -0.009(2) 0.014(3) C(4) 4a 0.7346(2) 0.0541(8) 0.5015(8) 0.053(3) 0.104(4) 0.104(5) 0.009(3) 0.001(3) 0.018(4) C(5) 4a 0.5643(1) 0.2231(7) 0.4165(6) 0.043(2) 0.054(3) 0.049(3) 0.004(2) -0.004(2) 0.004(2) C(6) 4a 0.5338(2) 0.3549(7) 0.5002(6) 0.061(3) 0.062(3) 0.047(3) 0.003(2) -0.001(2) 0.004(3) C(7) 4a 0.4844(1) 0.3039(8) 0.5060(6) 0.047(3) 0.094(4) 0.060(3) 0.012(3) 0.003(2) 0.005(3) C(8) 4a 0.4665(2) 0.1315(9) 0.4328(6) 0.050(3) 0.104(4) 0.068(4) -0.008(3) 0.004(3) 0.008(4) C(9) 4a 0.4967(2) -0.001(1) 0.3505(7) 0.059(3) 0.087(4) 0.078(4) -0.017(3) -0.006(3) -0.010(4) C(10) 4a 0.5457(2) 0.0458(8) 0.3437(6) 0.051(2) 0.073(3) 0.067(3) -0.004(3) 0.006(2) -0.006(3) C(ll) 4a 0.6307(2) 0.4104(8) 0.2695(6) 0.042(2) 0.055(3) 0.054(3) 0.008(2) -0.001(2) 0.005(2) C(12) 4a 0.6489(2) 0.6185(8) 0.2906(6) 0.059(3) 0.063(3) 0.065(3) 0.003(3) -0.001(3) 0.010(3) C(13) 4a 0.6614(2) 0.7396(8) 0.1667(7) 0.067(3) 0.069(3) 0.075(4) -0.003(2) 0.005(3) 0.016(4) C(14) 4a 0.6562(2) 0.661(1) 0.0232(7) 0.058(3) 0.100(5) 0.072(4) 0.005(3) 0.004(3) 0.033(4) C(15) 4a 0.6374(2) 0.461(1) 0.0000(7) 0.065(3) 0.120(5) 0.044(3) 0.008(3) 0.001(3) 0.013(4) C(I6) 4a 0.6245(1) 0.3344(8) 0.1237(6) 0.057(3) 0.077(3) 0.058(4) 0.005(2) -0.004(3) -0.001(3) C(17) 4a 0.7174(2) 0.4005(8) 0.6314(8) 0.068(3) 0.116(4) 0.091(5) -0.033(3) -0.013(4) 0.010(4) C(18) 4a 0.5524(2) 0.5442(8) 0.5852(7) 0.088(4) 0.083(4) 0.085(4) 0.002(3) 0.019(3) -0.016(4)
2. 3 f , £ D í a s t e r e o i i i e r
глС9 C18H19CI, monocUnic, P2\lc (No. 14), a =12.478(2) Â,
b =9.942(2) Â, с =12.632(3) Â, β =97.41(3)°, V=1554.0 Â^
Ζ =4, R(F) =0.059, R^F) =0.060.
Table 4. Parameters used for the X-ray data collection
Crystal: colorless prism, size 0.45 χ 0.55 χ 1.25 mm Wavelength: Mo Ka radiation (0.71073 λ )
μ: 2.30 cm-' Diffractometer: SYNTEX P3
Scan mode: Wyckoff
'^measurement' 2 9 3 К
29inax: 55°
щтшчяие·· 1758 Criterion for Fo. fo > 3 a(Fo)
^(param)refined· 172
Program: SHELXTL-pIus
Fig. 2. Molecule plot of the M, E diastereomer of С4Н4(СбН5)(С7Н7)С1СНз.
Diastereomers С4Н4(СбН5)(С7Н7)С1СНз
719
Table 5. Final atomic coordinates and displacement parameters (in Â^) Table 5. (Continued)
Atom Site ДГ v f/iso Atom Site X V •7 fiso
H(3) 4e 0.7149(3) 01156(4) 07385(3) 0.08 H(I4) 4e 0.7858(5) 0.7147(5) 0.3537(4) 0.08 H(4A) 4e 0.6744(4) -0.1005(5) 0.6761(4) 0.08 H(15) 4e 0.6350(4) 0.7015(5) 0.4437(4) 0.08 H(4B) 4e 0.7545(4) -0.0301(5) 0.6077(4) 0.08 H(16) 4e 0.6175(4) 0.5213(5) 0.5618(4) 0.08 H(4C) 4e 06312(4) -0.0398(5) 0.5642(4) 0.08 H(17A) 4e 0.5428(3) 0.0326(5) 0.7622(4) 0.08 H(7) 4e 09219(5) 0.4468(5) 0.9410(4) 0.08 H(17B) 4e 0.4969(3) 0.0987(5) 0.6530(4) 0.08 H(8) 4e 1.0702(5) 0.3117(7) 0.9277(5) 0.08 H(17C) 4e 0.5408(3) 0.1896(5) 0.7505(4) 0.08 H(9) 4e 1.0709(4) 0.1631(6) 0.7882(5) 0.08 H(I8A) 4e 07557(4) 0.5334(5) 0.8705(4) 0.08 H(IO) 4e 0.9283(3) 0.1663(5) 0.6453(4) 0.08 H(I8B) 4e 06812(4) 0.4277(5) 0.8048(4) 0.08 H(I2) 4e 0.9032(3) 0.3765(4) 0.4988(3) 0.08 H(I8C) 4e 0.7357(4) 0.5426(5) 0.7456(4) 0.08 H(I3) 4e 0.9165(4) 0.5506(5) 0.3786(4) 0.08
Table 6. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X У г t/ll 1/22 Í/33 Un l/i3 Í/23
CI 4e 0.58151(9) 0 77?7(1) 0.48794(9) 0.0836(8) 0.118(1) 0.0929(8) -0.0131(7) -0.0066(6) 0.0007(7) C d ) 4e 0.7516(3) 0.3169(4) 0.6167(3) 0.060(2) 0.066(3) 0.075(3) 0.004(2) 0.017(2) -0.006(2) C(2) 4e 0.6765(3) 0.2208(4) 0.6044(3) 0.068(2) 0.081(3) 0.076(3) 0.001(2) 0.011(2) -0.010(2) C(3) 4e 0.6618(3) 0.1066(4) 0.6769(3) 0.077(3) 0.084<3) 0.088(3) -0.015(2) 0.006(2) -0.002(3) C(4) 4e 0.6826(4) -0.0286(5) 0.6270(4) 0.115(4) 0.094(4) 0.118(4) -0.015(3) 0.008(3) 0.006(3) C(5) 4e 0.8390(3) 0.3114(4) 0.7095(3) 0.063(2) 0.069(3) 0.078(3) -0.013(2) 0.014(2) -0.002(2) C(6) 4e 0.8382(4) 0.3931(4) 0.7968(4) 0.089(3) 0.072(3) 0.089(3) -0.014(3) 0.020(3) 0.002(3) C(7) 4e 0.9233(5) 0.3917(5) 0.8787(4) 0.126(4) 0.109(4) 0.087(3) -0.042(4) -0.008(3) 0.005(3) C(8) 4e 1.0088(5) 0.3083(7) 0.8734<5) 0.094(4) 0.145(6) 0.112(4) -0.054(4) -0.024(3) 0.048(4) C(9) 4e 1.0111(4) 0.2237(6) 0.7879(5) 0.064(3) 0.138(5) 0.134(5) 0.001(3) 0.007(3) 0.049(4) C(10) 4e 0.9271(3) 0.2247(5) 0.7057(4) 0.066(3) 0.103(3) 0.102(3) 0.004(3) 0.010(2) 0.015(3) C ( l l ) 4e 0.7580(3) 0.4300(4) 0.5414(3) 0.062(2) 0.070(3) 0.078(3) 0.005(2) 0.010(2) -0.010(2) C(12) 4e 0.8466(3) 0.4423(4) 0.4869(3) 0.070(3) 0.072(3) 0.094(3) 0.005(2) 0.016(2) 0.001(3) C(13) 4e 0.8552(4) 0.5458(5) 0.4172(4) 0.094(3) 0.087(3) 0.102(3) -0.004<3) 0.027(3) 0.004<3) C(14) 4i 0.7789(5) 0.6418(5) 0.4022(4) 0.123(4) 0.079(3) 0.099(3) -0.002(3) 0.010(3) 0.011(3) C(15) 4e 0.6900(4) 0.6335(5) 0.4548(4) 0.111(4) 0.082(4) 0.127(4) 0.034(3) 0.010(3) 0.009(3) C(16) 4e 0.6795(4) 0.5275(5) 0.5244(4) 0.086(3) 0.098(4) 0.107(3) 0.027(3) 0.032(3) 0.011(3) C(17) 4e 0.5504(3) 0.1062(5) 0.7146(4) 0.093(3) 0.133(4) 0.111(4) -0.029(3) 0.028(3) 0.008(3) C(18) 4e 0.7448(4) 0.4823(5) 0.8055(4) 0.154(5) 0.094(4) 0.102(3) -0.014(4) 0.037(3) -0.024(3)
References
I. Doht, U. M.: 1,2-Disubstituierte 2-Halogenstyrole. - Synthese und Stereo- chemie der 1,2-Eliminierung zu Alienen. Dissertation, Universität Würz- burg, Germany 1986.
2. Peters, K.; Peters, E.-M.; Doht, U. M.; Quast, H.: Ciystal structures of the diastereomeric 2-bromo-3-methy 1-1 -(2-iiiethylphenyl)-1 -phenyl- 1-bute- nes. С4Н4(СбН5)(С7Н7)В1СНз. Ζ. Kristallogr. NCS 213 (1998) 720-722.
3. Sheldrick, G. M.: Program Package SHELXTL-plus. Release 4.1.
Siemens Analytical X-Ray Instruments Inc., Madison (WIS3719), USA 1990.