Zeitschrift für Kristallographie - New Crystal Structures 212, 1 7 9 - 1 8 0
© by R. Oldenbourg Verlag, München 1997
179
1 2
Crystal structure of (η -nitrato)^ -nitrato)(4-chlorophenylazophenyl) (triphenylphosphane)rhodium(III) dichloromethane,
(C30H23CIN4O6PRÍ1XCH2CI2)
U. R. Aulwurm, F. Knoch and H. Kisch
Universität Erlangen-Nürnberg, Institut für Anorganische Chemie II, Egerlandstr. 1, D-91058 Erlangen. Germany
Received July 16, 1996, CSD-No. 402568
Source of material: s e e ref. 1.
Table 1. Parameters used for the X-ray data collection
Crystal: brown platelets, size 0.7 χ 0.6 χ 0.4 mm Wavelength: Mo Ka radiation (0.71073 Â)
μ: 8.60 cm- 1
Diffractometer Siemens-Nicolet Scan mode: ω
^measurement- 2 9 3 Κ
20max: 54°
N(M/)U„„U,: 7214
Criterion for F0: F0> 4 a(F0)
N(param)refi„e<r. 4 1 5
Program: SHELXTL-plus
C3iH25Cl3N406PRh, triclinic, P I (No. 2), a =10.983(1) Â, b =12.184(2) Â, c =13.008(2) À, α =91.47(1)°, β =105.33(1)°, γ = 1 0 0 . 3 8 ( 1 ) ° , V=1646.4 À3, Ζ =2, R(F) =0.052, R^F) =0.049.
Table 2. Final atomic coordinates and displacement parameters (in Â2)
Atom Site X y ζ í/iso
H(1A) li 0.87480 0.74950 -0.05130 0.08 H(1B) li 0.87730 0.69590 0.05480 0.08 H(15) li 0.25530 0.36270 0.35910 0.08 H(14) 21 0.06660 0.41330 0.24180 0.08 H(13) 2 i 0.08550 0.52390 0.09040 0.08 H(12) li 0.29580 0.56490 0.05900 0.08 H ( l l ) 21 0.47690 0.52080 0.18160 0.08 H(24) li 0.90030 0.34460 0.49930 0.08 H(22) li 0.71790 0.26650 0.73630 0.08 H(21) li 0.52320 0.30880 0.63570 0.08 H(35) li 0.88350 0.27620 0.30220 0.08 H(34) li 1.05040 0.15820 0.36380 0.08 H(33) li 0.98100 -0.02700 0.38910 0.08 H(32) li 0.74910 -0.11680 0.32890 0.08 H(31) li 0.60230 -0.00430 0.28490 0.08 H(45) li 0.34940 0.15140 0.12040 0.08 H(44) li 0.16320 0.02480 0.16720 0.08 H(43) li 0.18550 -0.00390 0.35570 0.08 H(42) li 0.36800 0.04390 0.47620 0.08 H(41) li 0.57020 0.15170 0.43800 0.08 H(55) li 0.69220 0.11890 0.08700 0.08 H(54) li 0.63590 0.09400 -0.10430 0.08 H(53) li 0.47930 0.20560 -0.20160 0.08 H(52) li 0.38800 0.31970 -0.11500 0.08 H(51) li 0.44440 0.33960 0.07830 0.08
Table 3. Final atomic coordinates and displacement parameters (in Â2)
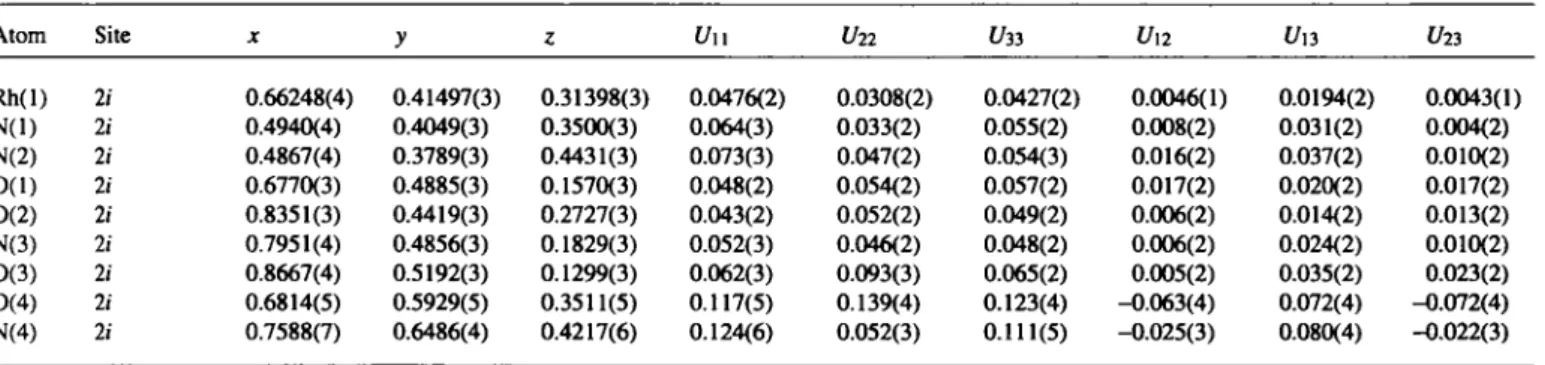
Atom Site X y ζ Un U22 ί/33 U\2 Un U23
Rh(l) li 0.66248(4) 0.41497(3) 0.31398(3) 0.0476(2) 0.0308(2) 0.0427(2) 0.0046(1) 0.0194(2) 0.0043(1) N(l) 2i 0.4940(4) 0.4049(3) 0.3500(3) 0.064(3) 0.033(2) 0.055(2) 0.008(2) 0.031(2) 0.004(2) N(2) 2 / 0.4867(4) 0.3789(3) 0.4431(3) 0.073(3) 0.047(2) 0.054(3) 0.016(2) 0.037(2) 0.010(2) 0(1) 21 0.6770(3) 0.4885(3) 0.1570(3) 0.048(2) 0.054(2) 0.057(2) 0.017(2) 0.020(2) 0.017(2) 0(2) li 0.8351(3) 0.4419(3) 0.2727(3) 0.043(2) 0.052(2) 0.049(2) 0.006(2) 0.014(2) 0.013(2) N(3) li 0.7951(4) 0.4856(3) 0.1829(3) 0.052(3) 0.046(2) 0.048(2) 0.006(2) 0.024(2) 0.010(2) 0(3) li 0.8667(4) 0.5192(3) 0.1299(3) 0.062(3) 0.093(3) 0.065(2) 0.005(2) 0.035(2) 0.023(2) 0(4) li 0.6814(5) 0.5929(5) 0.3511(5) 0.117(5) 0.139(4) 0.123(4) -0.063(4) 0.072(4) -0.072(4) N(4) li 0.7588(7) 0.6486(4) 0.4217(6) 0.124(6) 0.052(3) 0.111(5) -0.025(3) 0.080(4) -0.022(3)
180
(C30H23ClN4O6PRh)(CH2Cl2)Table 3. (Continued)
Atom Site X V ζ Un Un Í/3.1 Un U13 Un
0(5) li 0.7699(5) 0.7391(3) 0.4562(4) 0.133(4) 0.032(2) 0.094(3) -0.000(2) 0.032(3) -0.009(2) CX6) 2 i 0.8416(7) 0.5899(5) 0.4725(6) 0.212(8) 0.080(4) 0.211(8) 0.042(4) 0.123(6) 0.046(4) Cl(l) 2 i 0.9653(2) 0.2599(1) 0.6891(1) 0.108(1) 0.085(1) 0.069(1) 0.013(1) -0.010(1) 0.0218(9) C(15) 2 « 0.2612(6) 0.3971(5) 0.3010(5) 0.061(4) 0.062(3) 0.084(4) 0.003(3) 0.040(3) 0.007(3) C(14) li 0.1577(6) 0.4281(5) 0.2333(6) 0.048(4) 0.092(5) 0.106(5) 0.010(3) 0.035(4) 0.003(4) C(13) 2 i 0.1661(6) 0.4889(5) 0.1464(5) 0.049(4) 0.083(4) 0.085(4) 0.012(3) 0.018(3) -0.001(3) C(12) li 0.2830(5) 0.5197(5) 0.1284(4) 0.053(3) 0.069(3) 0.058(3) 0.009(3) 0.013(3) -0.001(3) 0(11) li 0.3916(5) 0.4921(4) 0.1954(4) 0.048(3) 0.052(3) 0.053(3) 0.012(2) 0.023(2) 0.005(2) C(10) li 0.3809(5) 0.4312(4) 0.2805(4) 0.054(3) 0.042(2) 0.060(3) 0.010(2) 0.023(3) -0.001(2) C(25) li 0.7036(5) 0.3612(4) 0.4588(4) 0.064(3) 0.037(2) 0.043(3) -0.001(2) 0.019(2) -0.002(2) C(24) li 0.8183(5) 0.3364(4) 0.5172(4) 0.061(3) 0.049(3) 0.044(3) -0.004(2) 0.006(3) 0.003(2) C(23) li 0.8237(6) 0.2975(4) 0.6168(4) 0.088(5) 0.047(3) 0.045(3) 0.010(3) 0.000(3) 0.002(2) C(22) li 0.7214(7) 0.2844(4) 0.6622(4) 0.115(6) 0.057(3) 0.042(3) 0.012(3) 0.029(3) 0.007(2) C(21) li 0.6090(6) 0.3139(4) 0.6060(4) 0.105(5) 0.056(3) 0.050(3) 0.014(3) 0.036(3) 0.008(3) C(20) li 0.6014(6) 0.3531(4) 0.5039(4) 0.086(4) 0.038(2) 0.048(3) 0.009(3) 0.029(3) 0.003(2) P(l) li 0.6098(1) 0.23270(9) 0.24437(9) 0.0365(6) 0.0310(5) 0.0411(6) 0.0075(5) 0.0118(5) 0.0034(5) C(35) li 0.8627(5) 0.1955(4) 0.3129(5) 0.046(3) 0.046(3) 0.100(5) 0.011(2) 0.018(3) 0.007(3) C(34) li 0.9529(6) 0.1302(5) 0.3478(6) 0.042(3) 0.074(4) 0.128(6) 0.022(3) 0.005(4) -0.001(4) C(33) li 0.9145(7) 0.0187(6) 0.3594(6) 0.075(5) 0.083(4) 0.101(5) 0.049(4) 0.005(4) 0.017(4) C(32) li 0.7881(7) -0.0289(5) 0.3343(6) 0.078(5) 0.053(3) 0.142(7) 0.027(3) 0.020(5) 0.024(4) C(31) li 0.6969(5) 0.0351(4) 0.3006(5) 0.056(4) 0.039(3) 0.115(5) 0.010(2) 0.022(3) 0.013(3) C(30) li 0.7320(5) 0.1491(3) 0.2892(4) 0.045(3) 0.037(2) 0.044(2) 0.012(2) 0.008(2) 0.003(2) C(45) li 0.3519(5) 0.1212(4) 0.1958(5) 0.045(3) 0.051(3) 0.102(5) 0.010(3) 0.021(3) 0.014(3) C(44) li 0.2458(6) 0.0606(5) 0.2222(8) 0.041(4) 0.068(4) 0.179(9) 0.001(3) 0.026(5) 0.020(5) C(43) li 0.2545(8) 0.0322(6) 0.3244(8) 0.080(5) 0.067(4) 0.186(9) 0.002(4) 0.084(6) 0.027(5) C(42) li 0.3693(8) 0.0637(5) 0.4024(6) 0.115(6) 0.063(4) 0.110(6) 0.003(4) 0.072(5) 0.021(4) C(41) li 0.4766(6) 0.1227(4) 0.3778(5) 0.081(4) 0.050(3) 0.072(4) -0.004(3) 0.042(3) 0.006(3) C(40) li 0.4670(4) 0.1527(3) 0.2734(4) 0.040(3) 0.030(2) 0.065(3) 0.006(2) 0.020(2) 0.003(2) C(55) li 0.6265(5) 0.1560(4) 0.0441(4) 0.069(4) 0.060(3) 0.051(3) 0.022(3) 0.019(3) -0.000(2) C(54) li 0.5935(6) 0.1475(5) -0.0655(5) 0.096(5) 0.074(4) 0.057(3) 0.028(4) 0.031(3) -0.007(3) C(53) li 0.5073(6) 0.2073(5) -0.1219(4) 0.093(5) 0.065(4) 0.043(3) -0.003(3) 0.014(3) -0.001(3) C(52) li 0.4527(5) 0.2755(4) -0.0696(4) 0.067(4) 0.055(3) 0.052(3) 0.009(3) 0.005(3) 0.010(2) C(51) li 0.4868(5) 0.2846(4) 0.0411(4) 0.053(3) 0.048(3) 0.047(3) 0.012(2) 0.006(2) 0.005(2) C(50) li 0.5730(4) 0.2236(3) 0.0992(4) 0.042(3) 0.038(2) 0.043(2) 0.007(2) 0.010(2) 0.001(2) C(l) li 0.9122(9) 0.7675(7) 0.0193(6) 0.144(8) 0.105(6) 0.093(6) -0.025(6) 0.011(6) 0.008(5) Cl(2) li 0.8854(2) 0.8822(2) 0.0785(2) 0.103(2) 0.136(2) 0.121(2) 0.025(1) -0.001(1) -0.009(1) Cl(3) li 1.0613(3) 0.7815(3) -0.0011(3) 0.116(2) 0.182(3) 0.247(4) -0.007(2) 0.071(2) -0.042(3)
Acknowledgements. This work was supported by Degssa AG and Fonds der Chemischen Industrie.
References
1. Aulwurm, U. R.: Rhodium-katalysierte Reaktionen von 1,2-Diaryldiazenen mit Diphenylacetylen. Dissertation, Universität Erlangen-Niirnberg, Ger- many 1996.
2. Sheldrick, G. M.: Program Package SHELXTL-plus. Release 4.1. Siemens Analytical X-Ray Instruments Inc., Madison (WI53719), USA 1990.