ζ . Kristallogr. NCS 213 (1998) 4 7 7 ^ 7 8 4 7 7
© by R. Oldenbourg Verlag, München
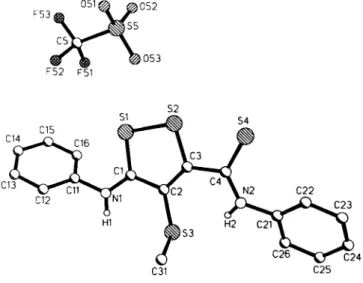
Crystal structure of 4-methylthio-3-(phenylamino)-5-(phenylamino- thiocarbonyl)-1,2-dithiol-1 -ium trifluoromethanesulfonate,
C4S3(NHC6H5)2(SCH3)(S03CF3)
K. Peters, E.-M. Peters
Max-Planck-Institut fur Festkörperforschung, HeisenbergstraBe I, D-70506 Stuttgart, Germany
E. Fanghänel and B. Kordts
Universität Halle-Wittenberg, Institut fur Organische Chemie, Geusaer Straße, D-06217 Merseburg, Germany Received September 12, 1997, CSD-No. 409087
Table 1. Parameters used for the X-ray data collection
0 5 3
C13
C24
Source of material: The title compound was obtained by stirring of 3-(pheny lamino)-5-(phenyIaminothiocarbonyl)-1,2-dithiol-1 - ium-4-thiolate (see ref. 1) with methyl triflate in 1,2-dichloro- ethane for Ih at room temperature. The precipitate was separated by suction and recrystallized from acetonitrile.
The starting material was wrongly described in ref. 1 as 3H,6H- 2,5-bis(phenyl)-1,2-thiazolino[5,4-i/] 1,2-thiazoline-3,5-dithione.
C 1 8 H 1 5 F 3 N 2 O 3 S 5 , triclinic, pT (NO. 2), a =10.211(4) Â, b =12.690(5) Â, с =9.546(4) Â, α =94.49(3)°, β =111.91(3)°, γ =96.13(3)°, V = 1 1 3 1 . 5 Â ^ Ζ =2, R(F) =0.054, Ry^F) =0.053.
Ciystal: dark red prism, size 0.4 χ 0.4 χ 0.6 mm Wavelength: Mo Kol radiation (0.71073 À)
μ: 5.60 cm"'
Difftactometer: Siemens P4
Scan mode: Wyckoff
Tmeosvnfffvm: 293 К
55°
5240 Criterion for Fo. Fo>3o(F„)
288
Program: SHFT.XTL-plus
Table 2. Final atomic coordinates and displacement parameters (in A^)
Atom Site X У ζ Uiso
H(l) 2/ 0.124(4) 0.606(3) 0.120(4) 0.06(1) H(2) 2i 0.230(4) 0.276(3) 0.327(4) 0.07(1) H(12A) 2i 0.1802(5) 0.8127(3) 0.2023(5) 0.08 H(13A) 2i 0.2203(6) 0.9674(4) 0.0946(7) 0.08 H(14A) 2i 0.2907(6) 0.9567(4) -0.1095(7) 0.08 H(15A) 2i 0.3106(5) 0.7933(4) -0.2186(6) 0.08 H(16A) 2i 0.2679(4) 0.6366(4) -0.1162(5) 0.08 H(22A) 2i 0.4812(5) 0.1492(3) 0.5816(5) 0.08 H(23A) 2i 0.4242(6) -0.0195(3) 0.6463(6) 0.08 H(24A) 2i 0.1959(6) -0.1135(3) 0.5265(6) 0.08 H(25A) 2i 0.0177(6) -0.0383(4) 0.3509(6) 0.08 H(26A) 2i 0.0722(5) 0.1300(3) 0.2841(6) 0.08 H(31A) 2i -0.0001(4) 0.4423(4) 0.3908(4) 0.08 H(31B) 2i 0.1110(4) 0.5416(4) 0.4034(4) 0.08 H(31C) 2i 0.1636(4) 0.4393(4) 0.4753(4) 0.08
Table 3. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X У ζ Un t/22 1/33 Un U,3 {/23
S(l) 2/ 0.46739(9) 0.59391(7) 0.1826(1) 0.0371(4) 0.0594(5) 0.0665(6) 0.0086(4) 0.0250(4) 0.0212(4) S(2) 2/ 0.55474(8) 0.47260(7) 0.3029(1) 0.0341(4) 0.0555(5) 0.0589(5) 0.0086(3) 0.0202(4) 0.0132(4) S(3) 2i 0.10160(8) 0.41201(7) 0.2165(1) 0.0330(4) 0.0648(5) 0.0516(5) 0.0029(3) 0.0174(3) 0.0113(4) S(4) 2i 0.5839(1) 0.29353(9) 0.4633(1) 0.0617(6) 0.0728(7) 0.0887(8) 0.0147(5) 0.0264(6) 0.0245(6) N(l) 2i 0.2001(3) 0.6175(2) 0.1177(3) 0.037(1) 0.058(2) 0.056(2) 0.010(1) 0.022(1) 0.015(1) N(2) 2i 0.3013(3) 0.2549(2) 0.3755(4) 0.044(2) 0.054(2) 0.074(2) 0.007(1) 0.023(2) 0.018(2) C(l) 2i 0.2992(3) 0.5579(2) 0.1761(4) 0.036(1) 0.051(2) 0.045(2) 0.006(1) 0.019(1) 0.007(1) C(2) 2i 0.2779(3) 0.4619(2) 0.2408(3) 0.034(1) 0.051(2) 0.042(2) 0.004(1) 0.018(1) 0.006(1)
478
C4S3(NHC6H5)2(SCH3)(S03CF3) Table 3. (Continued)Atom Site X ζ í/ii í/22 6-33 {/12 f/l3
C(3) 2/ 0.3960(3) 0.4150(2) 0.3058(4) 0.037(2) 0.046(2) 0.044(2) 0.003(1) 0.017(1) 0.004( 1 ) C(4) 2/ 0.4175(3) 0.3157(3) 0.3833(4) 0.046(2) 0.048(2) 0.048(2) 0.008(1) 0.020(1) 0.006(1) C(ll) 2/ 0.2242(3) 0.7130(3) 0.0535(4) 0.040(2) 0.059(2) 0.060(2) 0.013(1) 0.020<2) 0.020(2) C(12) 2i 0.2077(5) 0.8086(3) 0.1165(5) 0.083(3) 0.064(2) 0.081(3) 0.018(2) 0.044(2) 0.018(2) C(13) li 0.2319(6) 0.8994<4) 0.0529(7) 0.119(4) 0.063(3) 0.140(5) 0.028(3) 0.070(4) 0.030(3) C(14) 2i 0.2714(6) 0.8928(4) -0.0687(7) 0.123(5) 0.091(4) 0.151(6) 0.038(3) 0.081(4) 0.072(4) C(15) 2i 0.2846(5) 0.7971(4) -0.1317(6) 0.106(4) 0.115(4) 0.101(4) 0.046(3) 0.070(3) 0.061(3) C(I6) 2i 0.2600(4) 0.7049(4) -0.0715(5) 0.069(3) 0.084(3) 0.069(3) 0.029(2) 0.037(2) 0.029(2) C(21) 2i 0.2810(4) 0.1540(3) 0.4245(4) 0.062(2) 0.045(2) 0.071(2) 0.005(2) 0.036(2) 0.009(2) C(22) 2i 0.3862(5) 0.1108(3) 0.5332(5) 0.072(3) 0.064(2) 0.089(3) 0.010(2) 0.035(2) 0.026(2) C(23) 2i 0.3522(6) 0.0112(3) 0.5708(6) 0.103(4) 0.069(3) 0.102(4) 0.026(3) 0.056(3) 0.037(3) C(24) 2i 0.2173(6) -0.0437(3) 0.5013(6) 0.133(5) 0.050(2) 0.109(4) 0.003(3) 0.072(4) 0.016(3) C(25) 2i 0.1131(6) -0.0003(4) 0.3971(6) 0.098(4) 0.070(3) 0.110(4) -0.023(3) 0.043(3) 0.009(3) C(26) 2i 0.1454(5) 0.0992(3) 0.3580(6) 0.070(3) 0.069(3) 0.098(3) -0.004(2) 0.026(3) 0.020(2) C(3I) 2i 0.0931(4) 0.4649(4) 0.3919(4) 0.053(2) 0.113(3) 0.056(2) 0.008(2) 0.030(2) 0.010(2) S(5) 2i 0.86666(9) 0.67097(7) 0.2123(1) 0.0477(5) 0.0542(5) 0.0575(5) 0.0044(4) 0.0255(4) 0.0109(4) 0(51) 2i 0.9807(4) 0.7294(3) 0.3390(4) 0.103(3) 0.096(2) 0.091(2) 0.003(2) -0.014(2) 0.007(2) 0(52) 2i 0.9154(3) 0.6206(3) 0.1065(4) 0.085(2) 0.125(3) 0.087(2) 0.053(2) 0.046(2) 0.022(2) 0(53) 2i 0.7656(3) 0.6071(3) 0.2433(5) 0.058(2) 0.139(3) 0.186(4) 0.027(2) 0.063(2) 0.102(3) C(5) 2i 0.7709(5) 0.7716(3) 0.1088(5) 0.078(3) 0.064(2) 0.067(3) 0.018(2) 0.030(2) 0.014(2) F(51) 2i 0.6574(3) 0.7285(2) -0.0093(3) 0.094(2) 0.112(2) 0.084(2) 0.038(2) -0.001(2) 0.009(2) F(52) 2i 0.7294(4) 0.8328(3) 0.1913(4) 0.215(4) 0.121(3) 0.113(3) 0.107(3) 0.063(3) 0.008(2) F(53) 2i 0.8481(4) 0.8324(3) 0.0580(5) 0.135(3) 0.138(3) 0.214(4) 0.032(2) 0.081(3) 0.123(3)
References
1. Fanghänel, E.; Kordts, В.; Richter, Α. M.: Formation and isomerízation of 3íí,6H-2,5-bisaryl-l,2-tMazolino-[5,4-</]l,2-thiazoline-3,6-dithiones - derivates of a new heterocyclic system. Tetrahedron 45 (1989) 125-129.
2. Sheldrick, G. M.: Program Package SHELXTL-plus. Release 4.1.
Siemens Analytical X-Ray Instruments Inc., Madison (WI53719), US A 1990.
ζ. Kristallogr. NCS 213 (1998) 479-480 479
© by R. Oldenbourg Verlag, München
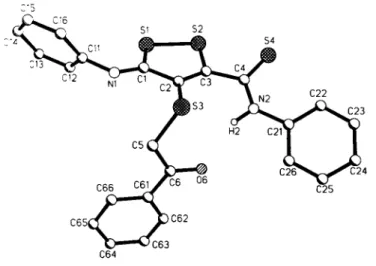
C r y s t a l s t r u c t u r e o f 3 H - 4 - ( p h e n a c y l t h i o ) - 5 - ( p h e n y l a m i n o t h i o c a r b o n y l ) - 3 - ( p h e n y I i m i n o ) - l , 2 . d i t h i o l , C 4 S 3 ( N H C 6 H 5 ) ( N C 6 H 5 ) ( S C H 2 C O C 6 H 5 )
K. Peters, E.-M. Peters
Max-Planck-lnstitut für Festkörperforschung. Heisenbergstraße 1, D-70506 Stuttgart, Germany E. Fanghänel and B. Kordts
Universität Halle-Wittenberg, Institut für Organische Chemie, Geusaer Straße, D-06217 Merseburg. Germany Received September 12. 1997. CSD-No. 409088
C23
C24
C64
Source of material: The title compound was obtained by refluxing of 3-(phenylamino-5-phenylaminothiocarbonyl)-1,2-ditMol-1 - ium-4-thiolate (see ref. 1 ) and phenacylbromide in chloroform for Ih. After cooling the product was separated by suction, stirred with water, separated and recrystallized from acetonitrile (see ref.
2).
The starting material was wrongly described in ref. 1 as
3H,6H-2,5-bis(phenyl)-1,2-thiazolino[5,4-ifl 1,2-thiazoline-3,5-dithione.
C24H18N2OS4, triclinic, P1 (NO. 2). a =10.253(6) Â,
b =13.027(6) Â, с =9.780(5) Â, α =90.86(4)°, β =117.83(4)°,
γ =98.48(4)°, ν=1137.3 Â^ Ζ=2, R(F} =0.048, R^F) =0.044.
Table 1. Parameters used for the X-ray data collection
Crystal: dark red plate, si7£ 0.35 χ 0.65 χ 0.2 nun Wavelength: Mo Ka radiation (0.71073 Â)
μ: 4.40 cm"'
Difftactometer: Siemens P4
Scan mode: Wyckoff
Тщ^ашгетеп/: 293 К
55°
ЩккОшид;^: 5253
Criterion for Fo: Fc>3c(Fo)
^(рагат)гфюГ· 284
Program: SHF.I.XTL-plus
Table 2. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X y ζ fiso
H(2) 2i H(5A) 2i H(5B) 2i H{12A) 2i H(13A) 2i H(14A) 2i H{15A) 2i H(16A) 2i H(22A) 2i H(23A) 2i H{24A) 2i H(25A) 2i H(26A) 2) H(62A) 2Í H(63A) 2i H(64A) 2i H(65A) 2/
H(66A) 2/
0.288(3) 0.1150(3) -0.0279(3) 0.4018(4) 0.4390(4) 0.3530(4) 0.2355(4) 0.1970(4) 0.4975(4) 0.4345(4) 0.2414(5) 0.1047(5) 0.1666(4) 0.1552(3) 0.0966(4) -0.0138(4) -0.0655(4) -0.0121(3)
0.272(2) 0.4513(2) 0.3828(2) 0.6684(3) 0.8310(3) 0.9716(3) 0.9521(2) 0.7909(2) 0.1824(3) 0.0145(3) -0.1068(3) -0.0618(3) 0.1040(2) 0.1427(2) 0.1150(3) 0.2348(3) 0.3835(3) 0.4134(3)
0.234(3) -0.0934(3) -0.1012(3) -0.1119(4) -0.1994(4) -0.1395(4) 0.0158(4) 0.1039(4) 0.6085(4) 0.6697(5) 0.4796(5) 0.2280(5) 0.1621(4) -0.2583(4) -0.5189(4) -0.6931(4) -0.6095(4) -0.3490(4)
0.062(9) 0.08 0.08 0.08 0.08 0.08 0.08 0.08 0.08 0.08 0.08 0.08 0.08
0.08
0.08 0.08 0.08 0.08
Table 3. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X y ζ í/ll U22 í/33 t/12 í/13 í/23
S(l) 2i 0.56494(9) 0.61547(6) 0.2110(1) 0.0583(5) 0.0595(5) 0.1131(7) -0.0022(4) 0.0446(5) 0.0138(5) S(2) 2i 0.64636(8) 0.50591(6) 0.3713(1) 0.0522(4) 0.0573(4) 0.0961(6) 0.0020(3) 0.0336(4) 0.0017(4) S(3) 2i 0.15877(8) 0.41506(5) 0.15199(9) 0.0535(4) 0.0472(4) 0.0877(5) 0.0034(3) 0.0436(4) 0.0020(4) S(4) 2i 0.64834(9) 0.33094(7) 0.5441(1) 0.0670(5) 0.0694(5) 0.0888(6) 0.0137(4) 0.0295(5) 0.0099(5) N(l) 2i 0.2659(3) 0.6161(2) 0.0570(3) 0.063(1) 0.046(1) 0.077(2) 0.004(1) 0.040(1) 0.005(1) N(2) 2i 0.3637(3) 0.2580(2) 0.3295(3) 0.068(2) 0.047(1) 0.071(2) 0.007(1) 0.034(1) 0.008(1) C(l) 2i 0.3727(3) 0.5685(2) 0.1439(3) 0.055(2) 0.044(1) 0.075(2) 0.002(1) 0.038(2) -0.002(1) C(2) 2i 0.3465(3) 0.4685(2) 0.2039(3) 0.052(2) 0.040(1) 0.075(2) 0.002(1) 0.039(1) -0.003(1)
480
C4S3(NHC6H5)(NC6H5)(SCH2C0C6H5) Table 3. (Continued)Atom Site X Ζ Un Ul2 Un Un Un Uli
C(3) 2/ 0.4681(3) 0.4315(2) 0.3085(3) 0.054(2) 0.043(1) 0.072(2) 0.003(1) 0.036(1) -0.005(1) C(4) 2Í 0.4828(3) 0.3338(2) 0.3923(3) 0.063(2) 0.048(1) 0.070(2) 0.011(1) 0.041(2) -0.002(1) C(5) 2/ 0.0791(3) 0.3915(2) -0.0565(3) 0.049(2) 0.048(2) 0.082(2) 0.004(1) 0.029(1) 0.003(1) C(6) 2i 0.1173(3) 0.2966(2) -0.1102(4) 0.058(2) 0.048(2) 0.080(2) 0.006(1) 0.036(2) 0.006(1) 0(6) 2i 0.1796(3) 0.2342(2) -0.0197(3) 0.132(2) 0.063(1) 0.086(2) 0.045(1) 0.054(2) 0.018(1) C(ll) 2i 0.2969(3) 0.7138(2) 0.0065(3) 0.063(2) 0.047(2) 0.068(2) -0.000(1) 0.035(2) 0.004(1) Cd 2) Ii 0.3673(4) 0.7262(3) -0.0850(4) 0.099(3) 0.064(2) 0.095(3) 0.015(2) 0.066(2) 0.009(2) C(13) Ii 0.3891(4) 0.8226(3) -0.1372(4) 0.126(3) 0.084(3) 0.109(3) 0.012(2) 0.087(3) 0.024(2) C(14) 2i 0.3393(4) 0.9055(3) -0.1014(4) 0.120(3) 0.059(2) 0.103(3) 0.001(2) 0.067(3) 0.019(2) C(15) 2i 0.2690(4) 0.8937(2) -0.0111(4) 0.136(3) 0.050(2) 0.105(3) 0.019(2) 0.076(3) O.Ol 1(2) C(16) 2i 0.2474(4) 0.7985(2) 0.0420(4) 0.105(3) 0.052(2) 0.094(2) 0.012(2) 0.068(2) 0.009(2) C(21) 2i 0.3382(3) 0.1577(2) 0.3788(4) 0.074(2) 0.048(2) 0.082(2) 0.015(1) 0.052(2) 0.012(1) C(22) 2i 0.4180(4) 0.1323(3) 0.5302(4) 0.071(2) 0.078(2) 0.095(3) 0.017(2) 0.047(2) 0.023(2) C(23) 2Í 0.3800(4) 0.0331(3) 0.5658(5) 0.093(3) 0.094(3) 0.119(3) 0.037(2) 0.069(3) 0.057(3) C(24) 2/ 0.2656(5) -0.0383(3) 0.4537(5) 0.115(3) 0.061(2) 0.155(4) 0.030(2) 0.099(3) 0.041(2) C(25) 2i 0.1858(5) -0.0121(3) 0.3052(5) 0.124(3) 0.051(2) 0.123(3) -0.001(2) 0.087(3) -0.001(2) C(26) 2i 0.2216(4) 0.0859(2) 0.2663(4) 0.107(3) 0.051(2) 0.084(2) 0.002(2) 0.062(2) 0.002(2) C(61) 2i 0.0783(3) 0.2811(2) -0.2769(4) 0.045(1) 0.055(2) 0.077(2) 0.003(1) 0.030(1) 0.006(1) C(62) 2i 0.1097(3) 0.1926(2) -0.3293(4) 0.067(2) 0.068(2) 0.092(2) 0.013(2) 0.047(2) 0.008(2) C(63) 2i 0.0751(4) 0.1763(3) -0.4834(4) 0.080(2) 0.089(3) 0.094(3) 0.015(2) 0.054(2) -0.005(2) C(64) 2i 0.0099(4) 0.2469(3) -0.5863(4) 0.082(2) 0.107(3) 0.079(2) 0.007(2) 0.043(2) 0.005(2) C(65) 2i -0.0211(4) 0.3341(3) -0.5373(4) 0.085(3) 0.090(3) 0.082(3) 0.013(2) 0.026(2) 0.021(2) C(66) 2/ 0.0113(3) 0.3523(3) -0.3826(4) 0.061(2) 0.068(2) 0.085(2) 0.008(2) 0.028(2) 0.006(2)
References
1. Fanghänel, E.; Kordts, В.; Richter, Α. M.: Formation and isomerization of 3ff,6//-2,5-bisaiyl-l,2-thiazolino[5,4-rf]l,2-thiazoline-3,6-dithiones - derívales of a new heterocyclic system. Tetrahedron 45 (1989) 125-129.
2. Kordts, В.: Synthese und Eigenschaften von 3//,6H-l,2-Dithiolo[4,3-c]- 1,2-dithiolderivaten. Dissertation, TH Merseburg, Germany 1990.
3. Sheldrick, G. M.: Program Package SHELXTL-plus. Release 4.1.
Siemens Analytical X-Ray Instruments Inc., Madison (WI53719), US A 1990.