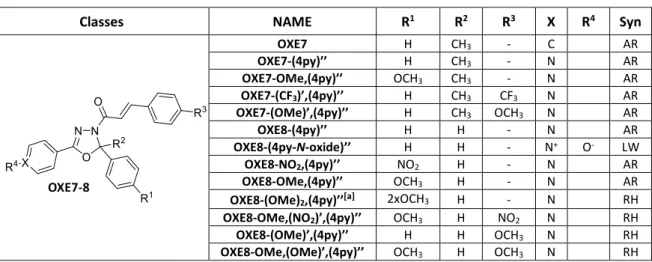
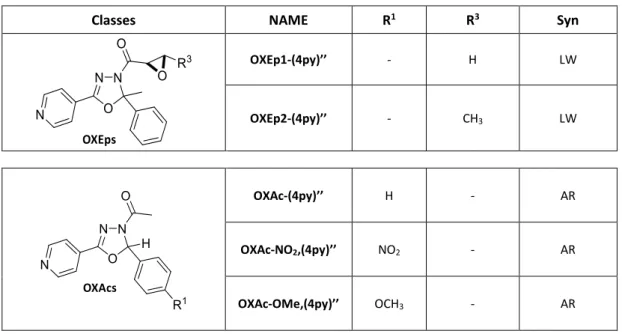
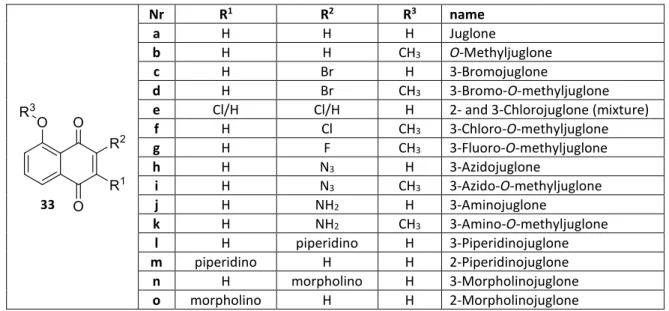
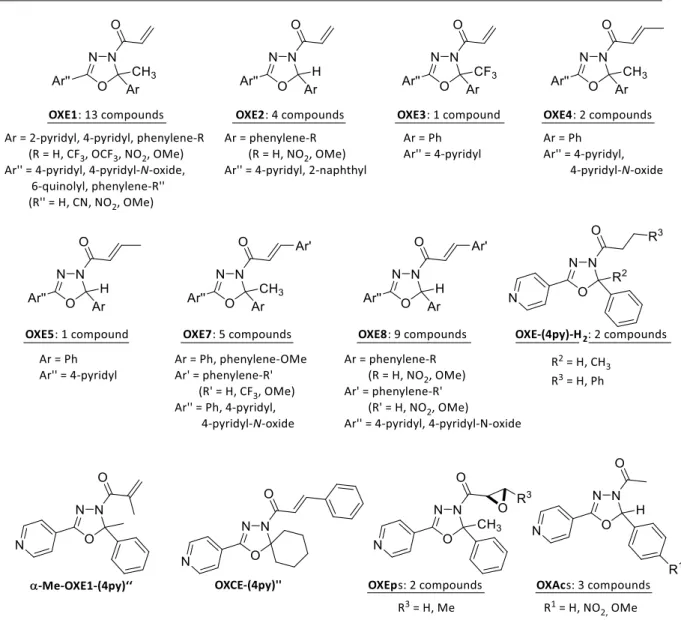
Reactivities of biologically active small molecules: Kinetic assessment of electrophilic enones and characterization of a photoactive phosphoantigen probe
Volltext
Abbildung
ÄHNLICHE DOKUMENTE
CCDC ID Formula Formula weight Z, calculated density F000 Description and size of crystal Absorption coefficient Min/max transmission Temperature Radiation wavelength Crystal
Midven- tral scale rows of this sample appear to show some geographical variation in this series, 34-37 on specimens (n = 6) from Kaptai and 38-42 on specimens (n = 5)
This two-compartment model consisted of a fast elimination and distribution phase (T 1/2 about 20 h) followed by a slow elimination phase (renal clearance about 0.11 ml/min.) and
The analyses did not find any explicit relationships for the last non-identifiable parameter, K i6Suc6P. However, it was found that the preceding measurements were suffi- cient to
The NH 4 + ion generates medium to strong hydrogen bonds with the car- bonylic oxygen, the iminic nitrogen and the sulfonyl oxygen atoms of the acesulfamate anion.. The FTIR spectrum
The Zn(II) cation in the [Zn(bispicen)Cl(H 2 O)] + complex is in a distorted octahedral environment, coordinated to a neu- tral bispicen molecule acting as a tetradentate ligand
Ogorodnyk Inorganic Chemistry Department, Kiev University, Volodymyrska Street 64, Kiev 01033, Ukraine Reprint requests to A.. The X-ray sin- gle crystal structure and
Key words: Phosphates, Crystal Structure, Langbeinite, Magnetic