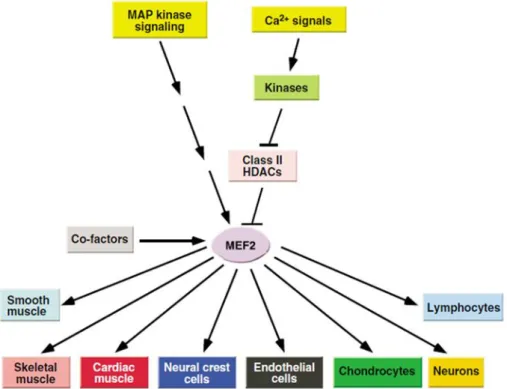
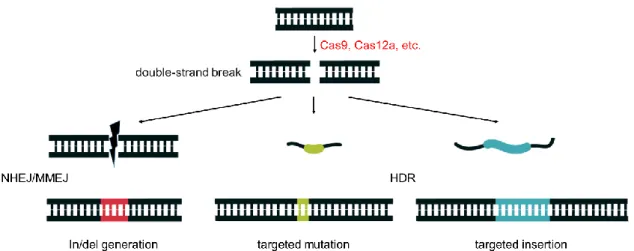
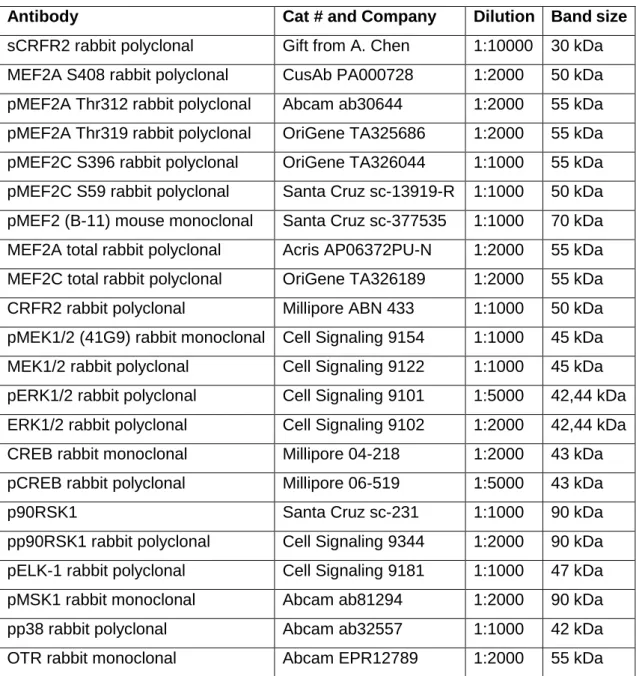
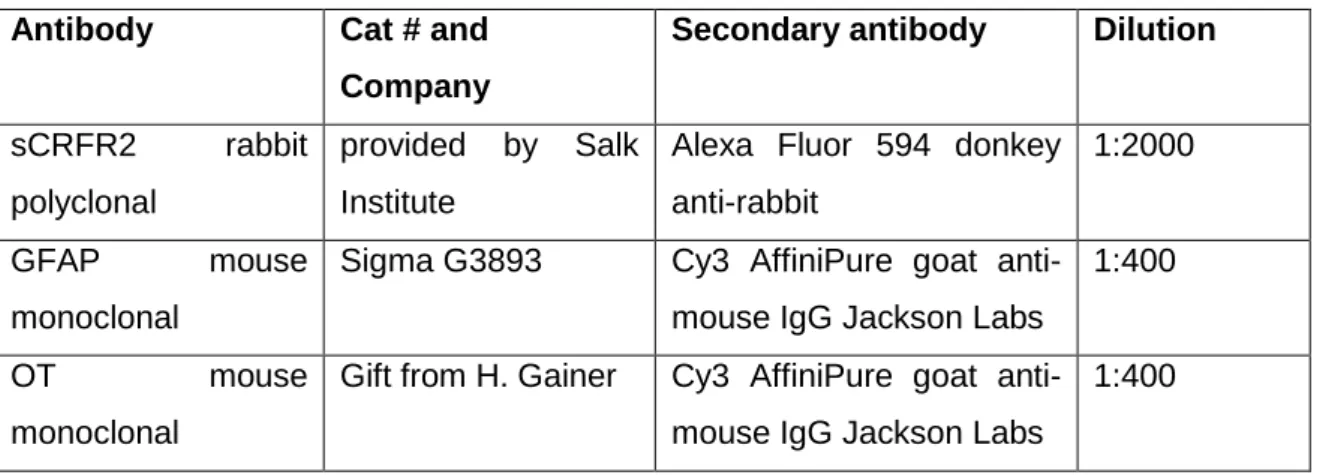
FROM INTRACELLULAR SIGNALING CASCADES TO BEHAVIOR: TOWARDS A BETTER UNDERSTANDING OF OXYTOCIN’S MOLECULAR EFFECTS
Volltext
Abbildung
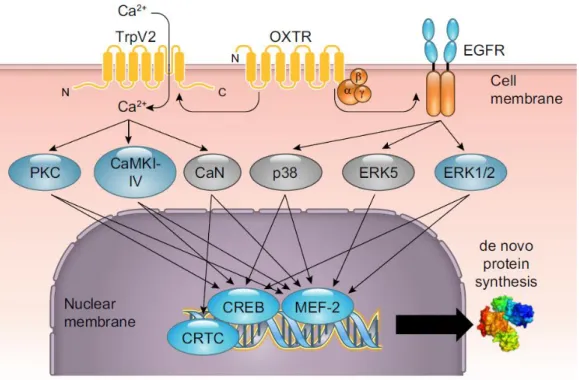
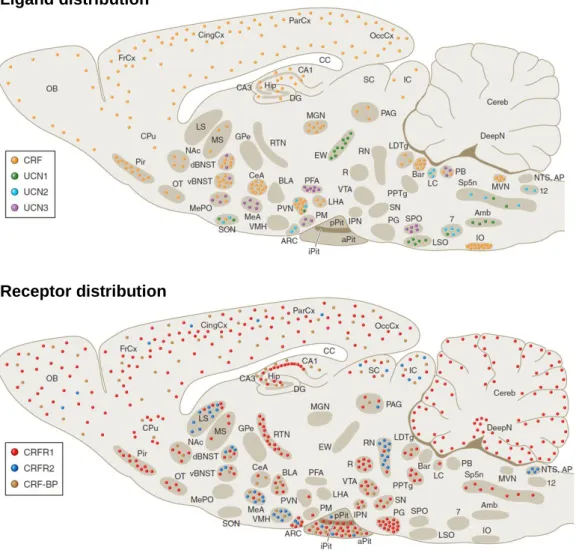
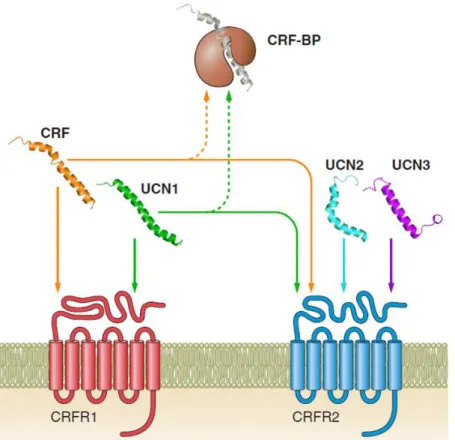
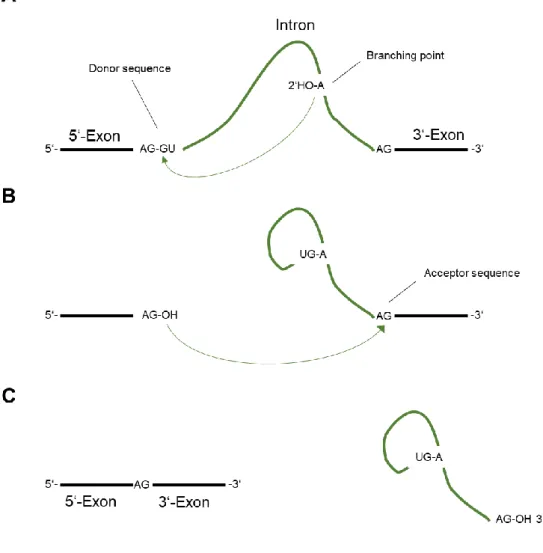
ÄHNLICHE DOKUMENTE
To investigate the effect of DLK on the recruitment of TORC to a CRE-containing promoter, a reporter gene assay (§3.15) was performed whereby a luciferase reporter gene controlled
Inhibition of HT1, in turn, enables the protein kinases OST1 or GHR1 to activate S-type anion channel SLAC1, which initiates ion efflux from guard cells and
Brognard, J., et al., Akt/protein kinase B is constitutively active in non- small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and
(2005) LmxMPK4, a mitogen-activated protein (MAP) kinase homologue essential for promastigotes and amastigotes of Leishmania mexicana. (2009) PubChem: a public information system
Three major kinases involved in the signal transduction cascade that restarts meiosis, are Maturation Promoting Factor (MPF), Mitogen-Activated Proteinkinase (MAPK)
In den Kumuluszellen allerdings wurde die p90rsk während der Reifung, sowohl in vitro als auch in vivo, nicht aktiviert und scheint somit dort keine Rolle als Substrat
The dependence of the steroid hormone synthesis in porcine cumulus cells of the MAPK (mitogen-activated protein kinase) activation and BMP6 (bone morphogenetic protein
ROS are thought to promote atherosclerosis through a variety of mechanisms, including enhanced oxidation of lipoproteins (Steinberg 1997), activation of proinflammatory genes (Marui,