Crystal structure of 5-(3-chlorobenzoyloxy)-4-ethoxycarbonyl-6,6- dimethyl-dihydro-1,3-oxazine 3-oxide, C 16 H 18 ClNO 6
Wolfgang Frey, Sunitha Shiva and Volker Jäger
*Universität Stuttgart, Institut für Organische Chemie, Pfaffenwaldring 55, 70569 Stuttgart, Germany Received June 2, 2010, accepted and available on-line June 20, 2010; CCDC no. 1267/3102
Abstract
C
16H
18ClNO
6, triclinic, P1 (no. 2), a = 8.5593(6) Å, b = 9.7225(4) Å, c = 11.3068(7) Å, * = 75.391(7)°, ) = 74.401(7)°, & = 81.440(7)°, V = 873.6 Å
3, Z = 2, R
gt(F) = 0.062, wR
ref(F
2) = 0.153, T = 293 K.
Source of material
The title compound was obtained starting from 5-ethoxy- carbonyl-5,5-dimethylisoxazoline [1-4]. N-Methylation with trimethyloxonium tetrafluoroborate [1-5] led to the 2-isoxazol- inium salt, which was deprotonated by means of triethylamine
[1,2,6-8] to afford the corresponding 3-isoxazoline ester. After attempted epoxidation with excess m-chlorobenzoic acid, the crude reaction product was purified by chromatography on silica, leading to colourless, analytically pure crystals of the title com- pound (m.p. 78 - 80 °C), with the unexpected nitrone structure identifiable only by crystal structure analysis [3,8-10].
Experimental details
H atoms were located on difference Fourier map, but refined with fixed individual displacement parameters using a riding model with d(C—H) ranging from 0.93 to 0.98 Å. In addition, the methyl groups were allowed to rotate but not to tip.
Discussion
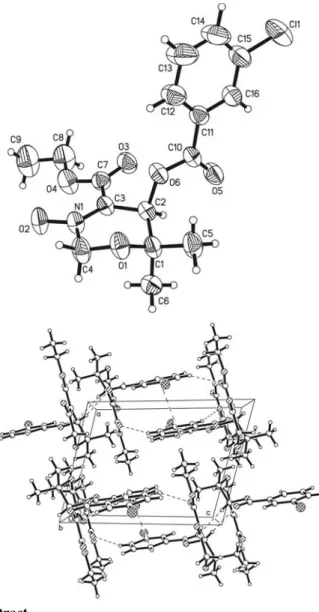
The title compound crystallizes with one independent molecule in the asymmetric unit (figure, top). The nitrone function is char- acterized by the N1—O1 distance of 1.271(3) Å, and the N1=C3 double bond is clearly identified by the distance of 1.316(3) Å.
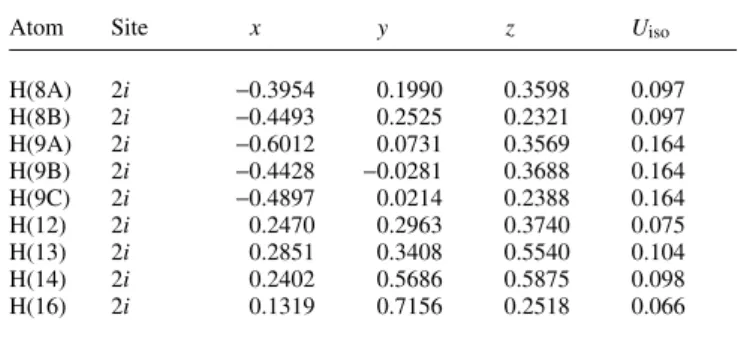
The oxazine ring shows a twist conformation, where N1, C2, C3 and C4 are in plane, C1 is 0.38(5) Å below and O1 is 0.35(5) Å above of this plane. The m-chlorobenzoyloxy moiety have a nearly perpendicular orientation to the oxazine ring signed by a interplanar angle of 84.2(1)°. The crystal packing is stabilized by a couple of weak inter-molecular electrostatic contacts (figure, bottom). First, the methyl group C6–H6A acts as donor to O2 of the nitrone function with a H6A···O2 distance of 2.44 Å and an angle C6–H6A···O2 of 167°. C14–H14 of the chlorobenzoyloxy moiety build up an electrostatic interaction to O3 of the ethyl ester with a H14···O3 distance of 2.55 Å and an angle C14–H14···O3 of 159°. C2–H2 act as donor with a distance H2···O5 of 2.48 Å to the carbonyl function O5 of the benzoyloxy moiety with an angle C2–H2···O5 of 133°. C16–H16 of the m-chlorobenzoyloxy moi- ety as donor builds up an electrostatic contact to O2 of the nitrone function with a distance H16···O2 of 2.50 Å and an angle C16–H16···O2 of 162°. Finally, the structure shows evidence of a ,-, interaction between the m-chlorobenzoyl moieties which causes an antiparallel face-to-face orientation. The distance be- tween the centers of the , systems is 3.67 Å.
Z. Kristallogr. NCS 225 (2010) 541-542 / DOI 10.1524/ncrs.2010.0237 541
© by Oldenbourg Wissenschaftsverlag, München
_____________
* Correspondence author (e-mail: jager.ioc@oc.uni-stuttgart.de)
Crystal: colorless plates, size 0.15 × 0.5 × 0.5 mm Wavelength: Cu K*radiation (1.54178 Å)
-: 22.18 cm−1
Diffractometer, scan mode: Siemens P4,%
2+max: 133.92°
N(hkl)measured, N(hkl)unique: 3600, 2995 Criterion for Iobs, N(hkl)gt: Iobs> 2((Iobs), 2344 N(param)refined: 221
Programs: SHELXS-97, SHELXL-97,
SHELXTL [11]
Table 1. Data collection and handling.
542 C16H18ClNO6
Cl(1) 2i 0.1434(2) 0.8343(1) 0.4496(1) 0.180(1) 0.0798(7) 0.1114(9) −0.0246(7) −0.0332(9) −0.0559(7) O(1) 2i 0.3653(2) 0.1286(2) 0.0199(2) 0.0377(9) 0.0385(9) 0.088(2) 0.0004(7) −0.0138(9) −0.0181(9) C(1) 2i 0.3016(3) 0.2699(3) −0.0350(3) 0.041(1) 0.035(1) 0.064(2) −0.005(1) −0.007(1) −0.016(1) N(1) 2i 0.0916(3) 0.0590(2) 0.1067(2) 0.042(1) 0.035(1) 0.063(1) −0.0048(9) −0.012(1) −0.013(1) O(2) 2i 0.0111(2) −0.0502(2) 0.1473(2) 0.055(1) 0.036(1) 0.092(2) −0.0112(8) −0.011(1) −0.013(1) C(2) 2i 0.1483(3) 0.3085(3) 0.0607(2) 0.043(1) 0.036(1) 0.050(1) −0.003(1) −0.016(1) −0.014(1) C(3) 2i 0.0379(3) 0.1908(3) 0.1161(2) 0.040(1) 0.036(1) 0.052(2) −0.002(1) −0.011(1) −0.016(1) O(3) 2i −0.1655(2) 0.3502(2) 0.2051(2) 0.059(1) 0.049(1) 0.069(1) −0.0003(9) −0.002(1) −0.026(1) O(4) 2i −0.2338(2) 0.1340(2) 0.2144(2) 0.041(1) 0.059(1) 0.102(2) −0.0081(9) 0.005(1) −0.035(1)
C(4) 2i 0.2631(3) 0.0233(3) 0.0383(3) 0.041(1) 0.036(1) 0.088(2) 0.000(1) −0.004(1) −0.019(1)
O(5) 2i 0.1325(3) 0.5768(2) 0.0916(2) 0.082(1) 0.039(1) 0.066(1) −0.0009(9) −0.032(1) −0.0161(9) C(5) 2i 0.4366(4) 0.3644(3) −0.0560(3) 0.049(2) 0.052(2) 0.088(2) −0.016(1) −0.004(2) −0.024(2) O(6) 2i 0.1890(2) 0.3419(2) 0.1671(2) 0.054(1) 0.0368(9) 0.057(1) −0.0007(7) −0.0222(8) −0.0169(8) C(6) 2i 0.2606(4) 0.2754(3) −0.1588(3) 0.063(2) 0.055(2) 0.058(2) −0.006(1) −0.006(1) −0.021(1) C(7) 2i −0.1307(3) 0.2329(3) 0.1828(3) 0.044(1) 0.047(1) 0.049(2) −0.003(1) −0.008(1) −0.015(1) C(8) 2i −0.3987(4) 0.1722(4) 0.2835(4) 0.044(2) 0.073(2) 0.112(3) −0.006(2) 0.014(2) −0.031(2) C(9) 2i −0.4908(5) 0.0493(5) 0.3146(5) 0.065(2) 0.082(3) 0.158(5) −0.017(2) 0.027(3) −0.038(3) C(10) 2i 0.1653(3) 0.4808(3) 0.1742(3) 0.044(1) 0.039(1) 0.054(2) −0.005(1) −0.013(1) −0.018(1) C(11) 2i 0.1883(3) 0.5009(3) 0.2939(2) 0.040(1) 0.047(1) 0.048(1) −0.005(1) −0.005(1) −0.018(1) C(12) 2i 0.2320(4) 0.3892(4) 0.3855(3) 0.076(2) 0.060(2) 0.056(2) 0.007(2) −0.018(2) −0.022(2) C(13) 2i 0.2530(6) 0.4159(5) 0.4935(4) 0.120(3) 0.087(3) 0.060(2) 0.016(2) −0.036(2) −0.025(2) C(14) 2i 0.2274(5) 0.5516(5) 0.5135(4) 0.102(3) 0.098(3) 0.059(2) −0.009(2) −0.023(2) −0.038(2) C(15) 2i 0.1828(4) 0.6620(4) 0.4231(3) 0.079(2) 0.070(2) 0.066(2) −0.021(2) −0.008(2) −0.034(2) C(16) 2i 0.1623(4) 0.6398(3) 0.3125(3) 0.060(2) 0.052(2) 0.057(2) −0.013(1) −0.009(1) −0.019(1) Table 3. Atomic coordinates and displacement parameters (in Å2).
Atom Site x y z U11 U22 U33 U12 U13 U23
H(2) 2i 0.0882 0.3924 0.0194 0.050
H(4A) 2i 0.3060 −0.0650 0.0859 0.068
H(4B) 2i 0.2624 0.0077 −0.0430 0.068
H(5A) 2i 0.4660 0.3550 0.0219 0.094
H(5B) 2i 0.3996 0.4619 −0.0863 0.094
H(5C) 2i 0.5296 0.3362 −0.1171 0.094
H(6A) 2i 0.1733 0.2168 −0.1437 0.087
H(6B) 2i 0.3547 0.2408 −0.2154 0.087
H(6C) 2i 0.2279 0.3721 −0.1956 0.087
Table 2. Atomic coordinates and displacement parameters (in Å2).
Atom Site x y z Uiso
Acknowledgment. We are grateful to Altana Pharma (Prof. Dr. J. Senn- Bilfinger) for financial support of this work.
References
1. LeRoy, P.-Y.: Neue Reaktionen von Isoxazolinen und Isoxazolinium- Salzen: Reduktion, stereoselektive CC-Verknüpfung durch Addition von Nucleophilen. Synthese ungewöhnlicher Aminohydroxysäuren. Disserta- tion, Universität Stuttgart, 1997.
2. Henneböhle, M.: N-Methylisoxazolinium-Salze — Neue Bausteine in der stereoselektiven Synthese von Aminopolyolen, Aminolactonen und Aminosäuren. Dissertation, Universität Stuttgart, 2002.
3. Bathich, Y.: Synthesis of Hydroxy Amino Acids and Assignment of Con- figuration to Products of C-Nucleophile Addition of Isoxazolines and Isoxazolinium Salts. Dissertation, Universität Stuttgart, 2006.
4. Henneböle, M.; LeRoy, P.-Y.; Hein, M.; Ehrler, R.; Jäger, V.: Isoxazol- inium Salts in Asymmetric Synthesis. Stereoselective Reduction Induced by a 3*-Alkoxy Stereocentre. A New Approach to Polyfunctionalized*- Amino Acids. Z. Naturforsch. 59B (2004) 451-467.
5. Cerri, A.; De Micheli, C.; Gandolfi, R.: Dihydro-1,2-oxazole Derivatives.
Part IX1. Stereoselective Synthesis of Tetrahydro-1,2-oxazoles by So- dium Borohydride Reduction of 4,5-Dihydro-1,2-oxazolium Tetrafluoroborates. Synthesis (1974) 710-712.
6. Shatzmiller, S.; Shalom, E.; Lidor, R.; Tartkovski, E.: Synthese und Umsetzungen von 3-Isoxazolinen. Liebigs Ann. Chem. (1983) 906-912.
7. Shiva, S.: 3-Isoxazolines - An Unusual Class of Heterocyclic Enamines:
Promising Precursors Towards the Synthesis of Complex, Branched Amino Acids and Alcohols, 1,3-Oxazin-2-ones, and Pyrroles. Disserta- tion, Universität Stuttgart 2008.
8. Jäger, V.; Frey, W.; Bathich, Y.; Shiva, S.; Ibrahim, M.; Henneböhle, M.;
Imerhasan, M.: 2-Isoxazolinium Salts and 3-Isoxazolines: Exploratory Chemistry and Uses for the Synthesis of Branched Amino Polyols and Amino Acids. Z. Naturforsch. 65B (2010) in print.
9. Shiva, S.; Bathich, Y.; Henneböhle, M.; LeRoy, P.-Y.; Jäger, V.: 7.
Iminium-Salz-Tagung, Bartholomä (Germany). Book of Abstracts, 2005, p. 141.
10. Ibrahim, M.; Shiva, S.; Jäger, V.: 8. Iminium-Salz-Tagung, Bartholomä (Germany). Book of Abstracts, 2007, p. 137-138.
11. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112-122.
H(8A) 2i −0.3954 0.1990 0.3598 0.097
H(8B) 2i −0.4493 0.2525 0.2321 0.097
H(9A) 2i −0.6012 0.0731 0.3569 0.164
H(9B) 2i −0.4428 −0.0281 0.3688 0.164
H(9C) 2i −0.4897 0.0214 0.2388 0.164
H(12) 2i 0.2470 0.2963 0.3740 0.075
H(13) 2i 0.2851 0.3408 0.5540 0.104
H(14) 2i 0.2402 0.5686 0.5875 0.098
H(16) 2i 0.1319 0.7156 0.2518 0.066
Table 2. Continued.
Atom Site x y z Uiso