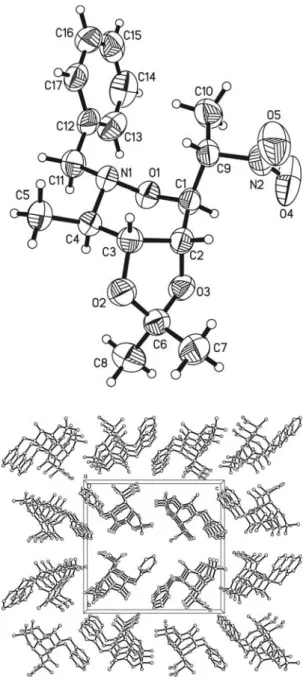
Crystal structure of (3R,4R,5R,6S,1'R)-2-benzyl-3-methyl-4,5-O-iso- propylidene-dioxy-6-(1'-nitroethyl)-tetrahydro-1,2-oxazine, C 17 H 24 N 2 O 5
Wolfgang Frey, Hua Yang and Volker Jäger
*Universität Stuttgart, Institut für Organische Chemie, Pfaffenwaldring 55, 70569 Stuttgart, Germnay Received June 9, 2010, accepted and available on-line July 23, 2010; CCDC no. 1267/3112
Abstract
C
17H
24N
2O
5, orthorhombic, P2
12
12
1(no. 19), a = 5.461(1) Å, b = 17.508(4) Å, c = 18.562(3) Å, V = 1774.9 Å
3, Z = 4, R
gt(F) = 0.049, wR
ref(F
2) = 0.135, T = 293 K.
Source of material
The title compound was obtained by reaction of (Z)-4,5-dideoxy- 2,3-O-isopropylidene-D-erythro-4-pentenose N-benzyl nitrone with nitroethane in tetrahydrofuran/chloroform in the presence of tetrabutylammonium fluoride trihydrate. The product was puri- fied by MPLC to give a spectrocopically and analytically pure, crystalline compound, m.p. 104 °C,[ ] *
D20= –13 (c = 0.60, CHCl
3).
Experimental details
H atoms were located on difference Fourier map, but refined with fixed individual displacement parameters using a riding model with d(C—H) = 0.93 - 0.98 Å. In addition, the methyl groups were allowed to rotate but not to tip. The oxygen atoms of the nitro function show enlarged displacement parameters perpendicular to the [N–O–O]-plane of the nitro group, which is a typical vibra- tional behaviour. The measured range of h k l is possibly not suit- able for characterisation of the absolute structure.
Discussion
The title compound crystallizes with one independent molecule in the asymmetric unit (figure, top). The oxazine ring shows a chair conformation and the dioxolane moiety has an envelope conformation where C2 is out-of-plane. A weak intramolecular electrostatic interaction is established between the methyl group C10 as donor and C12 of the ,-system of the phenyl group as ac- ceptor. The H10B···C12 distance is 2.80 Å with an angle C10–H10B···C12 of 152°. There is also evidence of a weak intermolecular electrostatic contact between C1–H1 of the oxazine ring as donor and the oxygen atom O5 of the nitro func- tion. The H1···O5 distance is 2.61 Å and the angle C1–H1···O5 is 154°. The antiparallel orientation in the packing organization (figure, bottom) of the molecules builds up hydrophobic channels generated by the benzyl moieties along [100].
Z. Kristallogr. NCS 225 (2010) 589-590 / DOI 10.1524/ncrs.2010.0257
589© by Oldenbourg Wissenschaftsverlag, München
Crystal: colorless block, size 0.5 × 0.6 × 0.9 mm Wavelength: Mo K*radiation (0.71073 Å)
-: 0.93 cm−1
Diffractometer, scan mode: Nicolet P3, Wyckoff
2+max: 60°
N(hkl)measured, N(hkl)unique: 5818, 5186 Criterion for Iobs, N(hkl)gt: Iobs> 2((Iobs), 4025 N(param)refined: 220
Programs: SHELXS-97, SHELXL-97,
SHELXTL-Plus [4]
Table 1. Data collection and handling.
_____________
* Correspondence author (e-mail: jager.ioc@oc.uni-stuttgart.de)
590
C
17H
24N
2O
5O(1) 4a −0.0980(2) 0.29230(7) 0.75701(6) 0.0576(7) 0.0599(7) 0.0489(6) 0.0032(6) 0.0028(6) 0.0059(5) N(1) 4a 0.0723(3) 0.24683(8) 0.80180(7) 0.0649(9) 0.0509(7) 0.0425(6) −0.0036(7) −0.0038(6) 0.0078(6) C(1) 4a 0.0325(3) 0.32425(9) 0.69799(9) 0.0606(9) 0.0487(8) 0.0422(7) 0.0070(7) 0.0017(7) 0.0063(6) O(2) 4a 0.2313(3) 0.13419(7) 0.64217(7) 0.0720(8) 0.0525(6) 0.0590(7) 0.0134(6) −0.0088(7) −0.0050(5) C(2) 4a 0.1449(3) 0.26258(9) 0.65108(8) 0.0598(9) 0.0486(8) 0.0437(7) 0.0020(7) 0.0015(7) 0.0039(6) N(2) 4a 0.3083(4) 0.42767(9) 0.6585(1) 0.082(1) 0.0496(8) 0.0608(9) −0.0003(8) 0.0112(9) 0.0076(7) O(3) 4a −0.0404(3) 0.22668(7) 0.60895(6) 0.0719(8) 0.0539(6) 0.0484(6) 0.0121(6) −0.0118(6) −0.0015(5) C(3) 4a 0.2502(3) 0.19542(9) 0.69249(9) 0.0533(9) 0.0485(8) 0.0532(8) 0.0042(7) −0.0063(7) −0.0004(7) C(4) 4a 0.1079(4) 0.17539(9) 0.76061(9) 0.066(1) 0.0471(8) 0.0479(8) −0.0049(8) −0.0110(8) 0.0041(6) O(4) 4a 0.1570(4) 0.4539(2) 0.6188(1) 0.111(2) 0.143(2) 0.110(2) 0.009(1) 0.004(1) 0.079(1) C(5) 4a 0.2495(5) 0.1164(1) 0.8042(1) 0.106(2) 0.056(1) 0.067(1) 0.005(1) −0.025(1) 0.0119(9) O(5) 4a 0.5261(4) 0.4363(1) 0.6492(1) 0.087(1) 0.108(2) 0.117(2) −0.016(1) 0.019(1) 0.031(1) C(6) 4a 0.0569(4) 0.1545(1) 0.5873(1) 0.068(1) 0.0519(9) 0.0495(8) 0.0057(8) −0.0032(8) −0.0016(7) C(7) 4a 0.1936(6) 0.1609(1) 0.5162(1) 0.108(2) 0.074(1) 0.054(1) 0.002(1) 0.013(1) −0.004(1) C(8) 4a −0.1493(4) 0.0977(1) 0.5852(2) 0.073(1) 0.068(1) 0.090(2) −0.005(1) −0.005(1) −0.011(1) C(9) 4a 0.2240(4) 0.3822(1) 0.72311(9) 0.073(1) 0.0452(8) 0.0506(8) 0.0003(8) 0.0026(8) 0.0062(7) C(10) 4a 0.1302(6) 0.4381(1) 0.7788(1) 0.128(2) 0.057(1) 0.063(1) −0.011(1) 0.019(1) −0.0081(9) C(11) 4a −0.0741(5) 0.2330(1) 0.8673(1) 0.091(1) 0.066(1) 0.0502(9) −0.018(1) 0.009(1) 0.0085(8) C(12) 4a −0.1203(4) 0.3039(1) 0.91151(9) 0.067(1) 0.069(1) 0.0407(7) −0.0122(9) 0.0078(8) 0.0111(7) C(13) 4a −0.3283(4) 0.3467(2) 0.9033(1) 0.067(1) 0.108(2) 0.059(1) −0.005(1) 0.000(1) 0.007(1) C(14) 4a −0.3667(5) 0.4108(2) 0.9461(2) 0.081(2) 0.104(2) 0.082(2) 0.025(2) 0.023(1) 0.019(1) C(15) 4a −0.1997(5) 0.4313(1) 0.9980(1) 0.106(2) 0.069(1) 0.062(1) −0.004(1) 0.025(1) 0.007(1) C(16) 4a 0.0075(5) 0.3886(1) 1.0067(1) 0.093(2) 0.079(1) 0.0494(9) −0.016(1) −0.000(1) 0.0018(9) C(17) 4a 0.0466(4) 0.3249(1) 0.9641(1) 0.069(1) 0.071(1) 0.0486(8) −0.002(1) −0.0009(9) 0.0125(8) Table 3. Atomic coordinates and displacement parameters (in Å2).
Atom Site x y z U11 U22 U33 U12 U13 U23
H(1) 4a −0.0863 0.3519 0.6682 0.061
H(2) 4a 0.2703 0.2847 0.6196 0.061
H(3) 4a 0.4225 0.2050 0.7042 0.062
H(4) 4a −0.0520 0.1543 0.7473 0.064
H(5A) 4a 0.3878 0.1404 0.8266 0.115
H(5B) 4a 0.3052 0.0764 0.7728 0.115
H(5C) 4a 0.1447 0.0952 0.8405 0.115
H(7A) 4a 0.2571 0.1117 0.5030 0.118
H(7B) 4a 0.3263 0.1964 0.5213 0.118
H(7C) 4a 0.0836 0.1785 0.4794 0.118
H(8A) 4a −0.0873 0.0487 0.5708 0.115
H(8B) 4a −0.2710 0.1144 0.5514 0.115
Table 2. Atomic coordinates and displacement parameters (in Å2)
.
Atom Site x y z Uiso
H(8C) 4a −0.2214 0.0936 0.6323 0.115
H(9) 4a 0.3644 0.3546 0.7432 0.068
H(10A) 4a 0.2460 0.4789 0.7844 0.124
H(10B) 4a 0.1089 0.4123 0.8240 0.124
H(10C) 4a −0.0240 0.4586 0.7632 0.124
H(11A) 4a 0.0101 0.1958 0.8971 0.083
H(11B) 4a −0.2304 0.2111 0.8535 0.083
H(13) 4a −0.4443 0.3329 0.8691 0.093
H(14) 4a −0.5069 0.4402 0.9394 0.107
H(15) 4a −0.2274 0.4739 1.0269 0.095
H(16) 4a 0.1225 0.4023 1.0413 0.088
H(17) 4a 0.1870 0.2958 0.9709 0.076
Table 2. Continued
.
Atom Site x y z Uiso
Acknowledgment. We are grateful to Prof. Dr. J. Senn-Bilfinger, Altana Pharma (now Nycomed), Konstanz, for financial support.
References
1. Yang, H.: Nucleophilic Additions to&,'-Unsaturated Nitrones: An Ap- proach to Functionalized Pyrrolidines and Tetrahydro-1,2-oxazines. Syn- thesis of 3-Phenylisothreonines: Analogues of the C-13 Side-Chain of Taxotere by Means of Chiral Borane Reagents. Dissertation, Universität Stuttgart, 2010.
2. Palmer, A. M.: Die Cope-House-Cyclisierung ungesättigter Hydroxylamine - ein neuer Weg zu Strukturen mit Glykosidase- Hemmaktivität. Dissertation, Universität Stuttgart, 2001.
3. Palmer, A. M.; Jäger, V.: Pyrrolidine N-Oxides by Stereoselective Addi- tion of Grignard and Lithium Compounds to 4,5-Dideoxy-2,3-O- isopropylidene-D-erythro-4-pentenose N-Benzyl Nitrone and Subsequent Cope-House Cyclization. Eur. J. Org. Chem. (2001) 1293-1308.
4. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112-122.