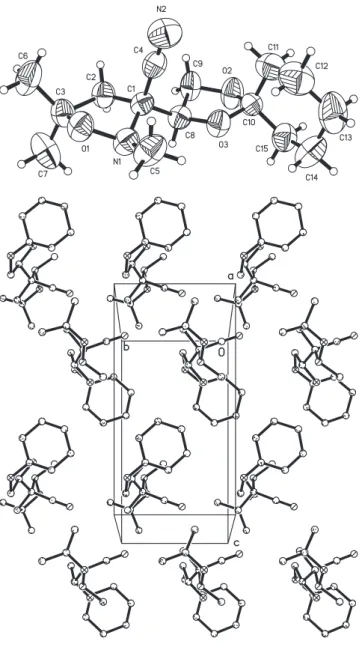
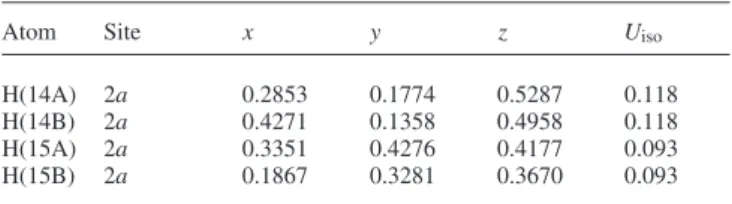
Crystal structure of (3S,1'S)-3-(1',2'-O-cyclohexylidenedioxyethyl)- 2,5,5-trimethyl-tetrahydro-1,2-oxazole-3-carbonitrile, C 15 H 24 N 2 O 3
Wolfgang Frey, Mohammad Ibrahim and Volker Jäger
*Universität Stuttgart, Institut für Organische Chemie, Pfaffenwaldring 55, 70569 Stuttgart, Germany Received June 23, 2008, accepted and available on-line September 25, 2008; CCDC no. 1267/2405
Abstract
C
15H
24N
2O
3, monoclinic, P2
1(no. 4), a = 9.718(2) Å,
b = 6.729(2) Å, c = 12.792(2) Å, 0 = 105.10(1)°, V = 807.6 Å
3, Z = 2, R
gt(F) = 0.078, wR
ref(F
2) = 0.157, T = 293 K.
SSource of material
The title compound has been obtained by nucleophilic addition of sodium cyanide NaCN to the N-methylisoxazolinium salts that
formed by N-methylation of (1'S)-3-(1',2'-O-cyclohexylidene- dioxyethyl)-5,5-dimethyl-4,5-dihydroisoxazole with Meerwein’s salt Me
3OBF
4[1-5]. Separation of the two diastereo- isomers, purification by column chromatography (petroleum ether/ethyl acetate) and crystallization from petroleum ether gave the title isoxazole-compound in the form of colourless crystals [1]
(m.p. 365 - 367 K); [2]
D20= −126.8 (c = 1.000, CHCl
3).
Discussion
The nitrile function C4−N2 was clearly characterized by the dis- tance of 1.147(7) Å. The isoxazolidine ring system shows an en- velope conformation, where the nitrogen N1 is out-of-plane (figure, top). The dioxolane moiety also shows an envelope con- formation, where the methylene group C9 is out-of-plane. Polar and non-polar layers are formed in the a,b plane stacking along [001]. One non-polar layer is established by the cyclohexyl moi- eties and the other one by the dimethyl groups. The polar layers are built up by the isoxazolidine rings (figure, bottom).
Z. Kristallogr. NCS 223 (2008) 453-454 / DOI 10.1524/ncrs.2008.0199
453© by Oldenbourg Wissenschaftsverlag, München
Crystal: colorless needles,
size 0.10 × 0.10 × 0.85 mm Wavelength: Mo K2radiation (0.71073 Å)
': 0.80 cm−1
Diffractometer, scan mode: Nicolet P3, Wyckoff scan
2#max: 50°
N(hkl)measured, N(hkl)unique: 3007, 2833 Criterion for Iobs, N(hkl)gt: Iobs> 2"(Iobs), 1829 N(param)refined: 182
Programs: SHELXS-97 [6], SHELDXL-97 [7]
SHELXTL-Plus [8]
Table 1. Data collection and handling.
H(2A) 2a 0.6707 0.5621 0.1296 0.074
H(2B) 2a 0.6563 0.3837 0.0474 0.074
H(5A) 2a 0.7430 0.1117 0.4033 0.124
H(5B) 2a 0.9051 0.1035 0.4062 0.124
H(5C) 2a 0.7934 −0.0122 0.3163 0.124
H(6A) 2a 0.8967 0.4311 −0.0039 0.140
H(6B) 2a 0.8670 0.2127 0.0269 0.140
H(6C) 2a 1.0167 0.3092 0.0761 0.140
H(7A) 2a 0.9112 0.7035 0.1382 0.161
H(7B) 2a 1.0362 0.5843 0.2146 0.161
H(7C) 2a 0.9014 0.6403 0.2540 0.161
H(8) 2a 0.5715 0.5032 0.2829 0.068
H(9A) 2a 0.4061 0.5610 0.1277 0.084
H(9B) 2a 0.3903 0.3338 0.0957 0.084
H(11A) 2a 0.1775 0.0617 0.2159 0.104
H(11B) 2a 0.3176 0.0232 0.1799 0.104
Table 2. Atomic coordinates and displacement parameters (in Å2)
.
Atom Site x y z Uiso
_____________
* Correspondence author (e-mail: jager.ioc@oc.uni-stuttgart.de)
N(1) 2a 0.7920(4) 0.2809(7) 0.2895(3) 0.055(2) 0.076(3) 0.061(2) 0.018(2) 0.016(2) 0.004(3) C(1) 2a 0.6581(5) 0.2898(7) 0.2019(3) 0.049(3) 0.049(3) 0.053(3) 0.014(2) 0.015(2) −0.001(3) O(1) 2a 0.8975(3) 0.2555(6) 0.2275(3) 0.053(2) 0.083(2) 0.070(2) 0.022(2) 0.017(2) 0.010(2) C(2) 2a 0.6999(5) 0.4266(8) 0.1210(4) 0.054(3) 0.064(3) 0.068(3) 0.008(3) 0.017(2) 0.008(3) O(2) 2a 0.2940(4) 0.4072(6) 0.2091(3) 0.060(2) 0.100(3) 0.087(3) 0.017(2) 0.028(2) 0.030(3) N(2) 2a 0.5855(5) −0.0628(8) 0.1208(4) 0.103(4) 0.065(3) 0.090(3) 0.011(3) 0.023(3) −0.004(3) O(3) 2a 0.4905(3) 0.2485(5) 0.3127(3) 0.059(2) 0.075(2) 0.064(2) 0.006(2) 0.020(2) 0.010(2) C(3) 2a 0.8653(5) 0.4109(9) 0.1473(4) 0.048(3) 0.072(4) 0.075(3) 0.010(3) 0.013(2) 0.008(3) C(4) 2a 0.6157(5) 0.0936(8) 0.1547(4) 0.061(3) 0.058(3) 0.061(3) 0.012(3) 0.025(2) 0.004(3)
C(5) 2a 0.8099(6) 0.106(1) 0.3599(4) 0.087(4) 0.098(4) 0.062(3) 0.031(4) 0.019(3) 0.016(3)
C(6) 2a 0.9160(6) 0.334(1) 0.0531(5) 0.075(4) 0.136(7) 0.078(4) 0.032(4) 0.036(3) 0.019(4)
C(7) 2a 0.9347(6) 0.602(1) 0.1926(6) 0.064(4) 0.101(5) 0.152(6) −0.007(4) 0.018(4) 0.009(5) C(8) 2a 0.5370(5) 0.3809(7) 0.2431(4) 0.061(3) 0.050(3) 0.060(3) 0.005(3) 0.020(3) 0.000(3)
C(9) 2a 0.4033(5) 0.427(1) 0.1552(4) 0.055(3) 0.083(4) 0.078(4) 0.009(3) 0.028(3) 0.023(3)
C(10) 2a 0.3361(5) 0.2549(9) 0.2847(4) 0.045(3) 0.078(4) 0.056(3) −0.004(3) 0.012(2) −0.004(3) C(11) 2a 0.2805(7) 0.0555(9) 0.2413(5) 0.076(4) 0.089(5) 0.089(4) −0.002(4) 0.013(3) −0.015(4) C(12) 2a 0.3206(8) −0.104(1) 0.3234(6) 0.127(6) 0.062(4) 0.122(6) −0.004(4) 0.025(5) −0.002(4) C(13) 2a 0.2666(8) −0.056(1) 0.4225(6) 0.120(6) 0.102(6) 0.132(6) −0.006(5) 0.040(5) 0.055(5) C(14) 2a 0.3244(8) 0.145(1) 0.4684(5) 0.117(5) 0.118(6) 0.067(4) 0.020(5) 0.035(4) 0.012(4) C(15) 2a 0.2888(6) 0.3057(9) 0.3865(5) 0.076(4) 0.078(4) 0.086(4) 0.001(3) 0.033(3) 0.000(3) Table 3. Atomic coordinates and displacement parameters (in Å2).
Atom Site x y z U11 U22 U33 U12 U13 U23
454
C
15H
24N
2O
3Acknowledgment. We are grateful to German Academic Exchange Services (DAAD), Germany, for financial support (Ph. D. scholarship to M. Ibrahim).
References
1. Ibrahim, M.: (2008) unpublished results.
2. Henneböhle, M.; LeRoy, P.-Y.; Hein, M.; Ehrler, R.; Jäger, V.:
Isoxazolinium Salts in Asymmetric Synthesis. Stereoselective Reduction Induced by a 3’-Alkoxy Stereocentre. A New Approach to Polyfunctionalizedα-Amino Acids. Z. Naturforsch. 59b (2004) 451-467.
3. Henneböhle, M. Dissertation, N-Methylisoxazolinium-Salze - Neue Bau- steine in der stereoseleKtiven Synthese von Aminopolyolen, Aminolactonen und Aminosäuren, Universität Stuttgart, 2002.
4. LeRoy, P.-Y.: Dissertation, Neue Reaktionen von Isoxazolinen und Isoxazolinium-Salzen: Reduktion, stereoselective CC-Verknüpfung durch Addition von Nucleophilen. Synthese von ungewöhnlicher Aminohydroxysäuren, Universität Stuttgart, 1997.
5. Jäger, V.; Bathich, Y.; Shiva, S.; Li, F.; Ibrahim, M.; Henneböhle, M.;
LeRoy, P.-Y.; Imerhasan, M.: 2-Isoxazolinium Salts and 3-Isoxazolines:
Exploratory Chemistry and Uses for the Synthesis of Branched Amino Polyols and Amino Acids, 2nd International Conference on Heterocyclic Chemistry, Jaipur (India) December 16-19, 2006, p.1-18 (in press).
6. Sheldrick, G. M.: SHELXS-97. Program for the Solution of Crystal Struc- tures. University of Göttingen, Germany 1997.
7. Sheldrick, G. M.: SHELXL-97. Program for the Refinement of Crystal Structures. University of Göttingen, Germany 1997.
8. Sheldrick, G.M.: SHELXTL-Plus. Structure Determination Software Suite. Release 4.1. Siemens Analytical Systems Inc., Madison, Wiscon- sin, USA 1991
H(12A) 2a 0.4235 −0.1177 0.3452 0.126
H(12B) 2a 0.2803 −0.2291 0.2923 0.126
H(13A) 2a 0.1632 −0.0532 0.4022 0.140
H(13B) 2a 0.2975 −0.1584 0.4770 0.140
Table 2. Continued
.
Atom Site x y z Uiso
H(14A) 2a 0.2853 0.1774 0.5287 0.118
H(14B) 2a 0.4271 0.1358 0.4958 0.118
H(15A) 2a 0.3351 0.4276 0.4177 0.093
H(15B) 2a 0.1867 0.3281 0.3670 0.093
Table 2. Continued
.
Atom Site x y z Uiso