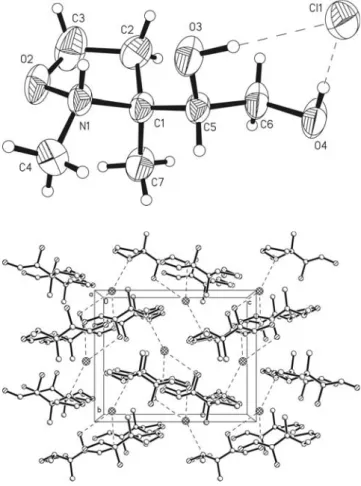
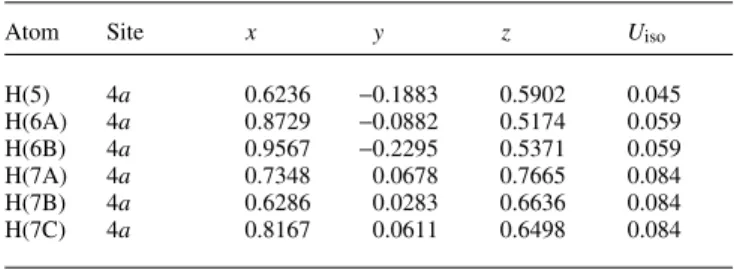
Crystal structure of (3S,1'S)-3-(1',2'-dihydroxyethyl)-2,3-dimethyl- tetrahydro-1,2-oxazolium chloride, [C 7 H 16 NO 3 ]Cl
Wolfgang Frey, Yaser Bathich, Marco Henneböhle and Volker Jäger
*Universität Stuttgart, Institut für Organische Chemie, Pfaffenwaldring 55, 70569 Stuttgart, Germany Received June 2, 2010, accepted and available on-line July 23, 2010; CCDC no. 1267/3101
Abstract
C
7H
16ClNO
3, orthorhombic, P2
12
12
1(no. 19), a = 8.1093(6) Å, b = 9.8455(6) Å, c = 12.1579(6) Å, V = 970.7 Å
3, Z = 4, R
gt(F) = 0.058, wR
ref(F
2) = 0.150, T = 293 K.
Source of material
The title compound was obtained from (1'S)-3-(1',2'-O- cyclohexylidenedioxyethyl)-2-methyl-4,5-dihydro-1,2-oxazol- ium tetrafluoroborate [1-4] by stereoselective addition of methyl- magnesium bromide in diethylether, followed by acetal hydroly- sis and treatment with 6 N hydrochloric acid. The crude product was purified by chromatography on silica and recrystallized form ethanol/petroleum ether to afford the hydrochloride as colourless crystals (m.p. 118 °C); [ ] *
D20= –60.8 (c = 1.00, MeOH).
Experimental details
H atoms were located on difference Fourier map, but refined with fixed individual displacement parameters using a riding model with d(C—H) and d(N—H) ranging from 0.91 to 0.98 Å, with ex- clusion of the hydrogen atoms of the hydroxy groups which are refined free, because of their relevance in hydrogen bond interac- tions. In addition, the methyl groups were allowed to rotate but not to tip. Only the so-called redundant data (–9 ≤ h ≤ 1, –11 ≤ k ≤ 1, –14 ≤ l ≤ 1) were collected because of the sensitivity of the crys- tal concerning decomposition. For the same reason, rapid data collection (scan speed ≥ 15° min/refl.) had to be done.
Discussion
The title compound crystallizes with one independent molecule in the asymmetric unit of the acentric space group P2
12
12
1(figure, top). The isoxazolidinium ring shows an envelope conformation where C1 is out of plane. The absolute configura- tion of the crystal structure is clearly determined by anomalous dispersion and in accordance with the synthetic investigations indicated by the Flack parameter x = –0.06(5). The view along [100] the packing diagram (figure, bottom) shows the chloride anion as threefold hydrogen bond acceptor. Both hydroxy functions (O3–H3, O4–H4) work as donors as well as the ionic nitrogen N1–H1. The H3···Cl1 distance is 2.21(7) Å and the angle O3–H3···Cl1 is 172(6)°. The hydroxy group O4–H4 also establishes a linear hydrogen bond to Cl1 with a H4···Cl1 distance of 2.08(9) Å and an angle C4–H4···Cl1 of 177(8)°. Finally, a weaker hydrogen bond is built up between N1–H1 and Cl1 with a H1···Cl1 distance of 2.33 Å and an angle N1–H1···Cl1 of 145°.
Z. Kristallogr. NCS 225 (2010) 585-586 / DOI 10.1524/ncrs.2010.0255
585© by Oldenbourg Wissenschaftsverlag, München
Crystal: orange block, size 0.4 × 0.4 5 0.5 mm Wavelength: Cu K*radiation (1.54178 Å)
-: 32.82 cm−1
Diffractometer, scan mode: Siemens P4,%
2+max: 135.86°
N(hkl)measured, N(hkl)unique: 1358, 1222 Criterion for Iobs, N(hkl)gt: Iobs> 2((Iobs), 1010 N(param)refined: 120
Programs: SHELXS-97, SHELXL-97, SHELXTL [6]
Table 1. Data collection and handling.
_____________
* Correspondence author (e-mail: jager.ioc@oc.uni-stuttgart.de)
H(1) 4a 0.6956 −0.2749 0.8166 0.048
H(2A) 4a 1.0273 −0.0856 0.7280 0.067
H(2B) 4a 0.9878 −0.2399 0.7513 0.067
H(3) 4a 0.727(9) −0.415(7) 0.580(6) 0.09(2) Table 2. Atomic coordinates and displacement parameters (in Å2)
.
Atom Site x y z Uiso
586
[C
7H
16NO
3]Cl
Cl(1) 4a 0.7466(2) −0.5404(1) 0.4302(1) 0.085(1) 0.0450(8) 0.0459(6) 0.0090(9) −0.0096(8) −0.0120(6) C(1) 4a 0.7767(7) −0.1281(5) 0.7157(4) 0.055(3) 0.033(2) 0.028(2) −0.002(2) −0.001(2) −0.003(2) N(1) 4a 0.6809(5) −0.1834(4) 0.8137(3) 0.058(2) 0.036(2) 0.026(2) 0.002(2) 0.002(2) −0.005(2) O(2) 4a 0.7553(6) −0.1245(4) 0.9082(3) 0.083(3) 0.057(2) 0.027(1) −0.010(3) 0.000(2) −0.010(2) C(2) 4a 0.9499(7) −0.1475(7) 0.7624(5) 0.057(3) 0.063(4) 0.048(3) −0.006(3) −0.012(3) −0.001(3) O(3) 4a 0.7342(7) −0.3517(3) 0.6471(3) 0.103(3) 0.029(2) 0.034(2) −0.006(2) 0.006(2) −0.001(1) C(3) 4a 0.9311(9) −0.1160(9) 0.8846(5) 0.074(4) 0.112(6) 0.047(3) −0.024(5) −0.011(3) −0.001(4) O(4) 4a 0.7930(6) −0.2309(4) 0.4186(3) 0.087(3) 0.054(2) 0.026(2) −0.002(2) 0.003(2) −0.001(2) C(4) 4a 0.5017(7) −0.1543(6) 0.8200(5) 0.059(3) 0.068(4) 0.052(3) 0.011(3) 0.012(3) −0.001(3) C(5) 4a 0.7350(7) −0.2126(4) 0.6145(3) 0.058(3) 0.028(2) 0.028(2) 0.000(3) 0.001(2) −0.002(2) C(6) 4a 0.8525(8) −0.1851(6) 0.5216(4) 0.072(4) 0.046(3) 0.029(2) −0.006(3) 0.009(2) −0.002(2)
C(7) 4a 0.735(1) 0.0210(5) 0.6972(4) 0.095(4) 0.030(2) 0.042(2) 0.002(4) 0.002(4) 0.000(2)
Table 3. Atomic coordinates and displacement parameters (in Å2).
Atom Site x y z U11 U22 U33 U12 U13 U23
H(3A) 4a 0.9918 −0.1813 0.9285 0.093
H(3B) 4a 0.9723 −0.0257 0.9009 0.093
H(4) 4a 0.78(1) −0.331(9) 0.422(7) 0.12(3)
H(4A) 4a 0.4574 −0.1934 0.8860 0.089
H(4B) 4a 0.4472 −0.1929 0.7573 0.089
H(4C) 4a 0.4846 −0.0578 0.8209 0.089
Table 2. Continued
.
Atom Site x y z Uiso
Acknowledgment. We are grateful to the Fonds der Chemischen Industrie for support of this work.
References
1. LeRoy, P.-Y.: Neue Reaktionen von Isoxazolinen und Isoxazolinium- Salzen: Reduktion, stereoselektive CC-Verknüpfung durch Addition von Nucleophilen. Synthese ungewöhnlicher Aminohydroxysäuren. Disserta- tion, Universität Stuttgart, 1997.
2. Henneböhle, M.: N-Methylisoxazolinium-Salze — Neue Bausteine in der stereoselektiven Synthese von Aminopolyolen, Aminolactonen und Aminosäuren. Dissertation, Universität Stuttgart, 2002.
3. Bathich, Y.: Synthesis of Hydroxy Amino Acids and Assignment of Con- figuration to Products of C-Nucleophile Addition of Isoxazolines and Isoxazolinium Salts. Dissertation, Universität Stuttgart, 2006.
4. Henneböhle, M.; LeRoy, P.-Y.; Hein, M.; Ehrler, R.; Jäger, V.: Isoxazol- inium Salts in Asymmetric Synthesis. Stereoselective Reduction Induced by a 3*-Alkoxy Stereocentre. A New Approach to Polyfunctionalized*- Amino Acids. Z. Naturforsch. 59B (2004) 451-467.
5. Jäger, V; Frey, W.; Bathich, Y.; Shiva, S.; Ibrahim, M.; Henneböhle, M.;
Imerhasan, M.: 2-Isoxazolinium Salts and 3-Isoxazolines: Exploratory Chemistry and Uses for the Synthesis of Branched Amino Polyols and Amino Acids. Z. Naturforsch. 65B (2010) 821-832.
6. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112-122.
H(5) 4a 0.6236 −0.1883 0.5902 0.045
H(6A) 4a 0.8729 −0.0882 0.5174 0.059
H(6B) 4a 0.9567 −0.2295 0.5371 0.059
H(7A) 4a 0.7348 0.0678 0.7665 0.084
H(7B) 4a 0.6286 0.0283 0.6636 0.084
H(7C) 4a 0.8167 0.0611 0.6498 0.084
Table 2. Continued
.
Atom Site x y z Uiso