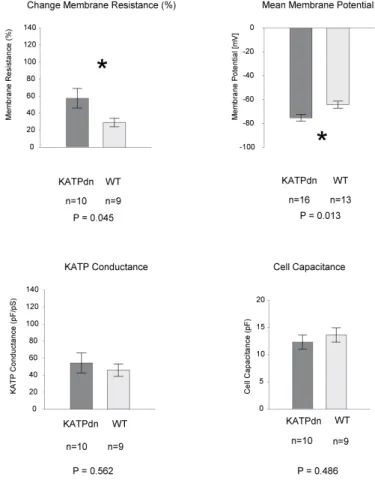
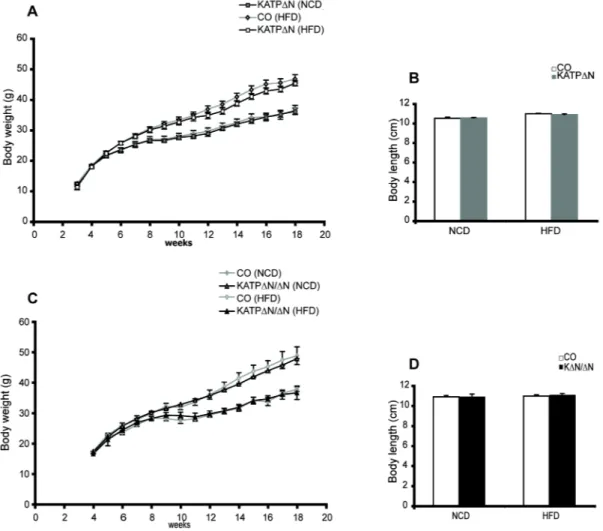
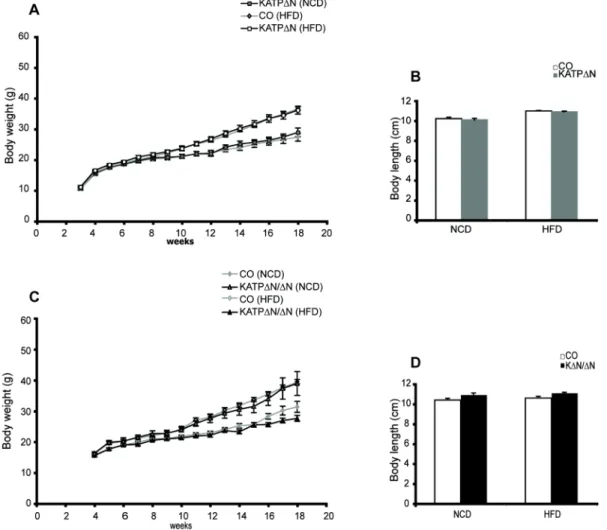
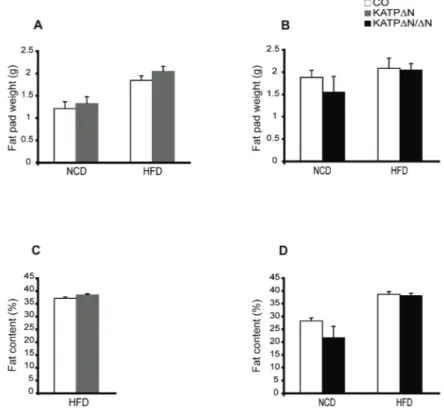
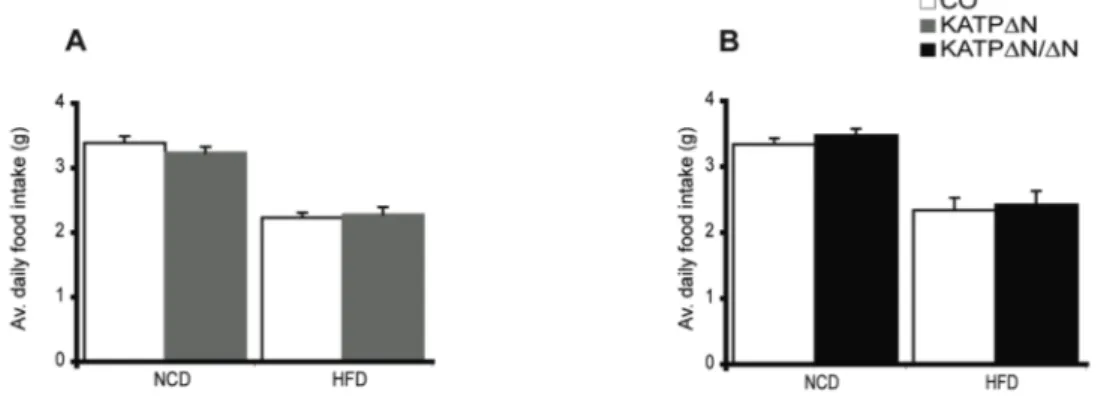
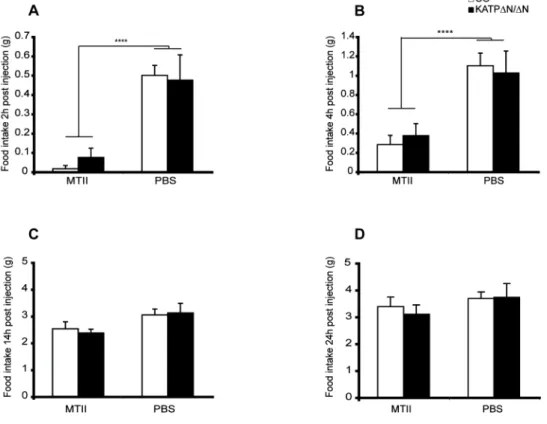
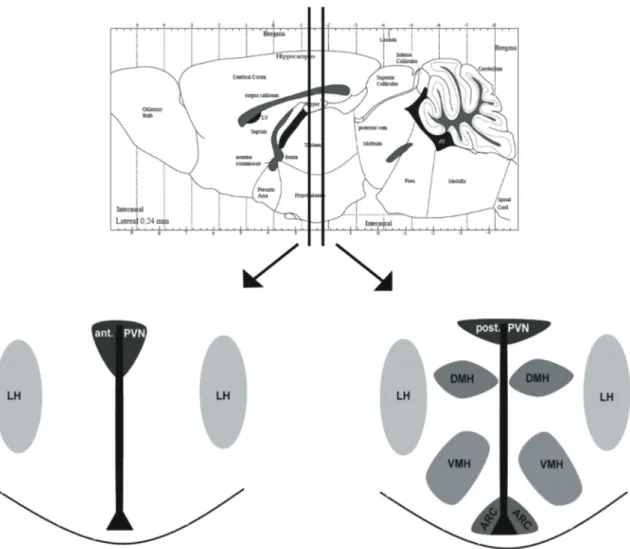
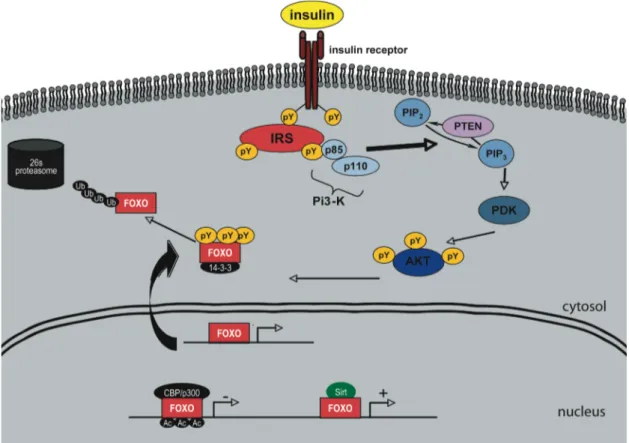
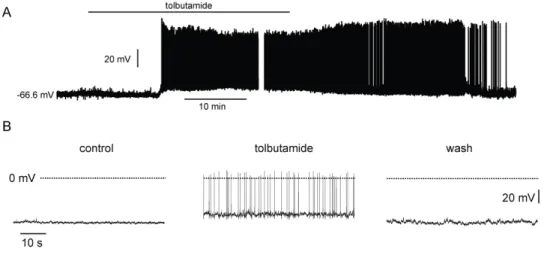
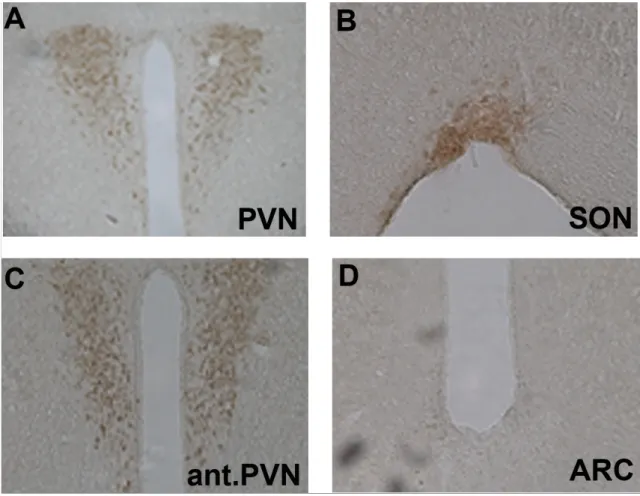
The role of FOXO1 and K ATP channel signalling in Sim1 expressing neurons for the regulation
Volltext
Abbildung
ÄHNLICHE DOKUMENTE
This note reports the results of semiempirical evaluations of C 6 for the five lowest energy levels of copper interacting with noble-gas atoms.. Applying the Eckart-Wigner theorem
circulating mf; 2 determine whether serum from singly infected hamsters during the prepatent, patent or latent stages, or hyperinfection serum would influence the
(b) In the dark region selection among the different win-stay, lose-shift strategies always leads to the fixation of an efficient strategy, whereas in the grey region some but not
Objectives The ratio of serum to tear concentration for a range of metabolites in ASED after prolonged storage time was determined to define dilution that maintains
The aim of the present thesis was to determine the effects of different impact factors (feed iodine supplementation E , iodine source E , RSC and poultry breed)
Targeted metabolomics analysis revealed (via measurement of acylcarnitines) that central insulin and leptin suppress long chain fatty acid β-oxidation in the liver
An axiomatization of the Public Good index for simple games was given in [Holler and Packel, 1983], so that some people also speak of the Holler–Packel index, and the generalization
Because RH421 reports the conversion of enzyme in the E1(Na þ ) 3 state to the E2P state, it was found (24) that the drop in ΔF/F 0 at high ATP concentrations (>20 μM) could