Research Collection
Journal Article
Transformation of TiO2 (nano)particles during sewage sludge incineration
Author(s):
Wielinski, Jonas; Voegelin, Andreas; Grobéty, Bernard; Müller, Christoph R.; Morgenroth, Eberhard; Kaegi, Ralf
Publication Date:
2021-06-05 Permanent Link:
https://doi.org/10.3929/ethz-b-000474262
Originally published in:
Journal of Hazardous Materials 411, http://doi.org/10.1016/j.jhazmat.2020.124932
Rights / License:
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International
This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use.
ETH Library
Journal of Hazardous Materials 411 (2021) 124932
Available online 5 March 2021
0304-3894/© 2020 The Author(s). Published by Elsevier B.V. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Transformation of TiO 2 (nano)particles during sewage sludge incineration
Jonas Wielinski a , b , 1 , Andreas Voegelin a , 2 , Bernard Grob ´ ety c , Christoph R. Müller d , 3 , Eberhard Morgenroth a , b , 4 , Ralf Kaegi a , * , 5
a
Eawag, Swiss Federal Institute of Aquatic Science and Technology, 8600 Dübendorf, Switzerland
b
ETH Zürich, Institute of Environmental Engineering, 8093 Zürich, Switzerland
c
University of Fribourg, Department of Geosciences, 1700 Fribourg, Switzerland
d
ETH Zürich, Department of Mechanical and Process Engineering, 8092 Zürich, Switzerland
A R T I C L E I N F O Editor: Prof. G. Lyberatos Keywords:
XAS Incineration Nano safety Sewage sludge ash Titanium dioxide Nanoparticle
A B S T R A C T
Titanium dioxide (TiO
2) (nano)particles are produced in large quantities and their potential impacts on eco- systems warrants investigations into their fate after disposal. TiO
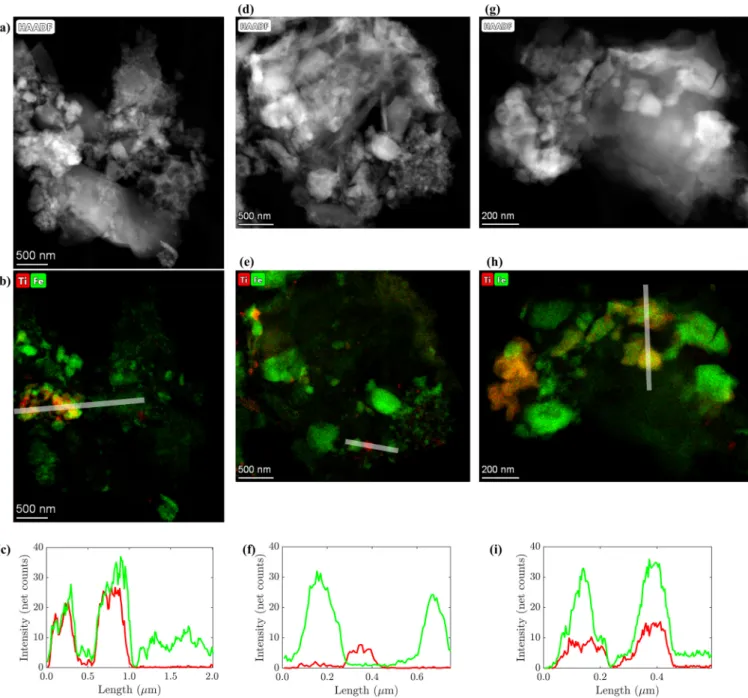
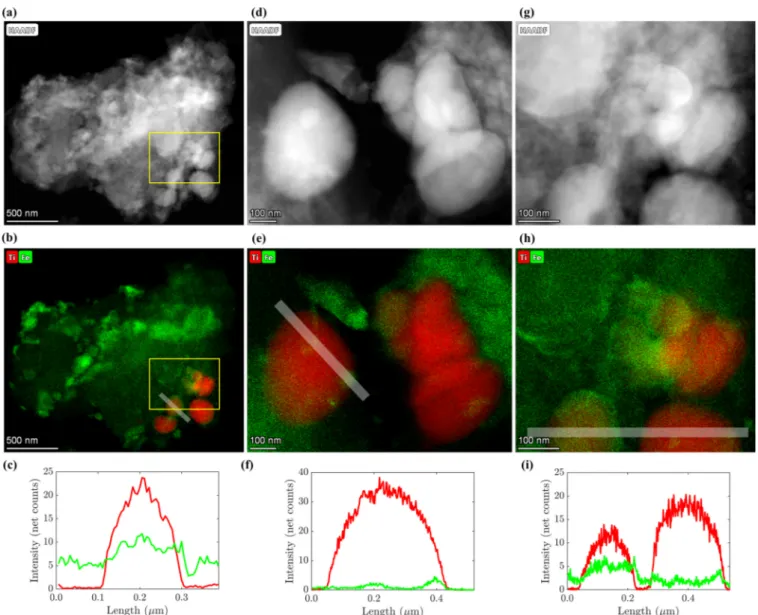
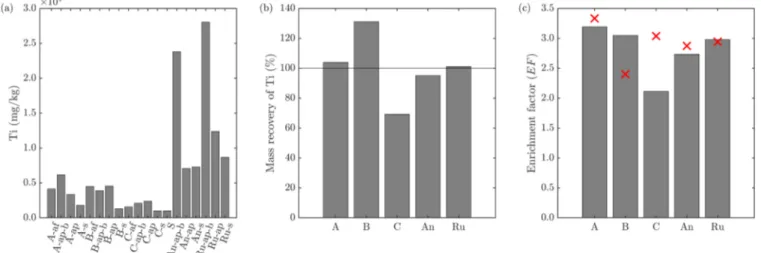
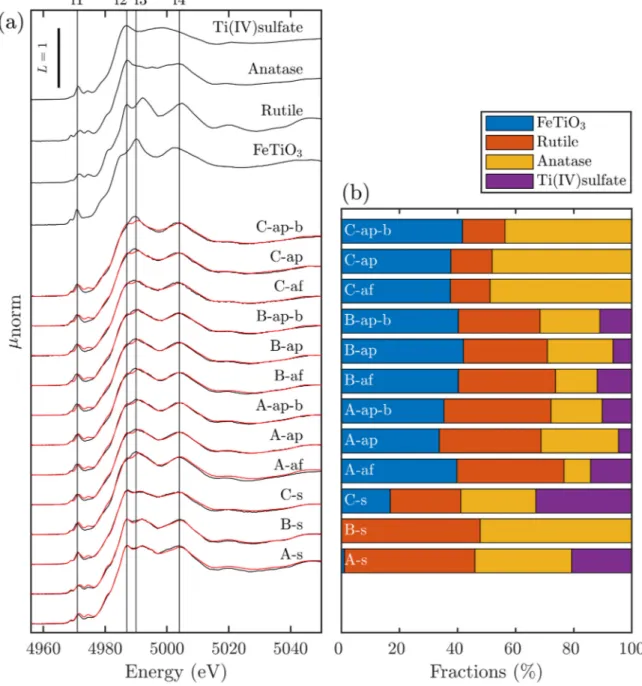
2particles released into wastewater are retained by wastewater treatment plants and accumulate in digested sludge, which is increasingly incinerated in indus- trialized countries. Therefore, we investigated the changes of the Ti-speciation during incineration of as-received sludge and of sludge spiked with anatase (d = 20 – 50 nm) or rutile (d = 200 – 400 nm) using X-ray absorption spectroscopy (XAS) and transmission electron microscopy (TEM). In the as-received sludge, rutile and anatase were the dominant Ti bearing minerals and both remained unaffected by the anaerobic treatment. During incineration, Ti reacts with hematite to members of the hematite-ilmenite solid solution series (Hem-Ilm). Up to 80% of the Ti spiked as anatase transformed into Hem-Ilm, a distorted 6-fold coordinated Ti (Ti(IV)sulfate) and rutile during incineration. Up to 30% and 60% of rutile transformed into Hem-Ilm and Ti(IV)sulfate represented phases in fly and bottom ash, respectively. Fe and Ti were spatially correlated in ash derived from as-received and anatase spiked sludge, whereas only a thin layer of the spiked rutile reacted with Fe, in line with XAS data. This study highlights the transient nature of nano-Ti species during sewage sludge incineration.
1. Introduction
Since more than a century, titanium dioxide (TiO
2) powders and nanoparticles (NP) have been used in industrial goods including paints and personal care products such as sunscreens. In 2019, the annual TiO
2production equaled roughly 7.66 million metric tons (USGS, 2020). TiO
2occurs as rutile, anatase and brookite with the latter polymorph being least abundant. Octahedra of six-fold O-coordinated Ti share corners in rutile and edges in anatase (Scheinost, 2005). In 2006, the International Agency for Research on Cancer (IARC) classified TiO
2-NP as potentially carcinogenic with regard to lung cancer in humans (class 2B) (Baan et al., 2006), and a proposal for a similar classification under European Union (EU) regulation is currently underway (Guseva Canu et al., 2019).
Furthermore, several studies document ecotoxicologial impacts of
(nano)TiO
2on aquatic organisms (Bundschuh et al., 2018; Lead et al., 2018; Du et al., 2019). Therefore, a detailed understanding of trans- formation processes in managed waste facilities that potentially impact the properties of TiO
2-NP is required to assess the potential risks asso- ciated with the use and disposal of TiO
2-NP (Svendsen et al., 2020).
Depending on the sewer system, TiO
2(nano)particles released from outdoor paints can either enter the natural aquatic environment or reach wastewater treatment plants (WWTPs) where they are retained and accumulate in the sewage sludge (Som et al., 2011; Lazareva and Keller, 2014; Kaegi et al., 2008). TiO
2-NP derived from cosmetic products like sunscreen mostly end up in sewage sludge. In high-income/industrialized countries, major shares of digested sewage sludge are incinerated (US, EU: 20 – 25%; Japan, Germany: > 50%;
Switzerland: close to 100%) (Werther and Ogada, 1999; Laube and
* Corresponding author.
E-mail address: ralf.kaegi@eawag.ch (R. Kaegi).
1
ORCID: 0000-0002-7017-3164
2
ORCID: 0000-0003-2873-8966
3
ORCID: 0000-0003-2234-6902
4
ORCID: 0000-0002-1217-269X
5

![Fig. 5. Ab initio calculated XANES spectra of (bottom to top) ulv ¨ ospinel (Ulv) (Wechsler et al., 1984), Ulv with 0.1% of total Ti in tetragonal sites (Ulv 0.1%[Ti 4+ ] (T) ) (Mudarra Navarro et al., 2019), ilmenite-substituted hematite (Ilm-sub Hem), h](https://thumb-eu.123doks.com/thumbv2/1library_info/3908301.1525907/9.892.259.629.334.1015/calculated-wechsler-tetragonal-mudarra-navarro-ilmenite-substituted-hematite.webp)