Z. Kristallogr. NCS 219 (2004) 403-404
© by Oldenbourg Wissenschaftsverlag, München
403
Crystal structure of l,4-bis(4,4-dimethylcyclohexa-2,5-dienylidene)- A^,A^',A^",A^"-tetra-o-tolylbut-2-ene-l,2,3,4-tetraamine, (C24H 2 2N 2 )2
R. D. Ernst*, R. Basta and A. M. Arif
University of Utah, Department of Chemistry, 315 S. 1400 E., Rm. 2020, Salt Lake City, Utah 84112-0850, USA Received July 2, 2004, accepted and available on-line October 8, 2004; CCDC no. 1267/1362
C24
Abstract _
C48H52N4, triclinic, PI (no. 2), a = 9.6328(2) A, b = 10.8348(3) A, c = 10.9423(3) A, a = 63.276(2)°, /? = 73.827(2)°,y = 82.635(1)°, V=979.7 A
3,Z= 1, Rg(F) = 0.030, wRnffF
2) = 0.136, T= 150 K.
Source of material
The compound was isolated as a byproduct of the reaction of o-tolylisocyanide with methylbromobis(6,6-dimethylcyclohexa- dienyl)zirconium [1,2] in hexane at 0 °C. Attempted crystalliza- tion of the metal-containing product by slow evaporation of an ether solution was not successful, but instead yielded a small amount of this hydrolysis product. The compound was crystal- lized by slow evaporation of solvent from an ether solution of the metal-containing product into diphenylmethane using an appara- tus previously described [3],
Discussion
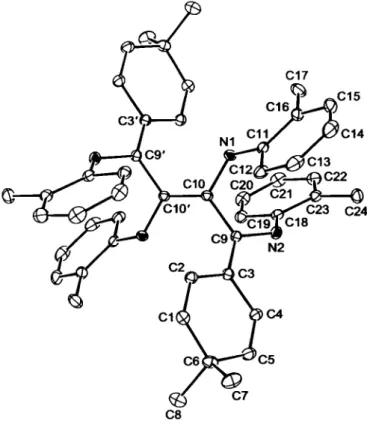
The structure consists of a highly conjugated, linear decapentaene backbone, with additional cross-conjugation due to vinyl groups on the C3 and C3' atoms, while amine groups are attached to C9,
C9', C10, and C10'. Throughout this framework one observes reasonable distances for both the expected single (1.456(2) A -
1.490(2) A) and double ( 1.332(2) A -1.364(3) A) carbon-carbon bonds. The most notable aspect of this structure is the significant twist about the central C=C bond out of a planar orientation with the two external cross-conjugated triene units. This appears to re- sult from intramolecular interactions between the six attached rings, and can be best exemplified by the C3-C9-C10-C10' tor- sion angle of-58.4(2)°.
Table 1. Data collection and handling.
Crystal:
Wavelength:
P-Diffractometer, scan mode:
20nax-
AW*0measured, APAOunk)«:
Criterion for /at», N(hkl)gc.
N(paramhtfined:
Programs:
yellow prism,
size 0.23 x 0.30 x 0.35 mm Mo Ka radiation (0.71073 Â) 0.68 cm- 1
Nonius KappaCCD, <p/a>
54.96°
7261,4438
/obs> lo(labs), 3230 340
SIR97 [4], SHELXL-97 [5]
Table 2. Atomic coordinates and displacement parameters (in Â2).
* Correspondence author (e-mail: ernst@chemistry.chem.utah.edu)
Atom Site X y z t/iso
H(l) 2 i 0.270(2) 0.355(2) 1.004(2) 0.049(5) H(1N) 2 i 0.720(2) 0.504(2) 0.377(2) 0.042(5) H(2) li 0.418(2) 0.435(2) 0.773(2) 0.040(4) H(2N) 2 i 0.594(2) 0.127(2) 0.609(2) 0.058(5) H(4) li 0.370(2) 0.052(2) 0.775(2) 0.041(4) H(5) li 0.227(2) -0.029(2) 1.006(2) 0.055(5) H(7A) li 0.385(3) 0.064(2) 1.156(2) 0.070(6) H(7B) li 0.228(2) -0.018(2) 1.235(2) 0.057(5) H(7C) li 0.240(2) 0.135(2) 1.236(2) 0.070(6) H(8A) li 0.031(2) 0.218(2) 1.137(2) 0.064(6) H(8B) 2i 0.013(2) 0.068(2) 1.130(2) 0.077(7) H(8C) li 0.024(2) 0.217(2) 0.983(2) 0.073(6) H(12) li 0.680(2) 0.369(2) 0.728(2) 0.047(5) H(13) li 0.855(2) 0.257(2) 0.855(2) 0.080(7) H(14) li 1.083(3) 0.214(2) 0.731(2) 0.080(7) H(15) li 1.125(3) 0.281(2) 0.484(2) 0.071(6) H(17A) li 0.963(2) 0.507(3) 0.248(2) 0.077(7) H(17B) li 0.890(2) 0.362(2) 0.279(2) 0.068(6) H(17C) li 1.058(3) 0.366(2) 0.276(2) 0.078(7) H(19) li 0.379(2) 0.362(2) 0.396(2) 0.044(5) H(20) li 0.394(2) 0.419(2) 0.157(2) 0.062(6) H(21) li 0.583(2) 0.311(2) 0.034(2) 0.056(5) H(22) li 0.742(2) 0.152(2) 0.158(2) 0.047(5) H(24A) li 0.825(2) 0.021(2) 0.367(2) 0.064(6) H(24B) li 0.710(2) -0.018(2) 0.505(2) 0.052(5) H(24C) li 0.821(2) 0.100(2) 0.463(2) 0.065(6)
404
( C 2 4 H22 N2) 2Table 3. Atomic coordinates and displacement parameters (in Â2).
Atom Site X y z U H 1/22 t/33 Un V ,3 i/23
N(I) 2i 0.6946(1) 0.4572(1) 0.4590(1) 0.0246(6) 0.0296(6) 0.0249(6) 0.0004(5) -0.0032(4) —0.0055(5) N(2) 2 i 0.5477(1) 0.2041(1) 0.5539(1) 0.0396(7) 0.0268(6) 0.0278(6) 0.0048(5) -0.0071(5) —0.0098(5) C(l) 2 i 0.2920(2) 0.2921(2) 0.9554(1) 0.0354(8) 0.0385(8) 0.0279(7) 0.0021(6) -0.0103(6) -0.0141(6) C(2) 2 i 0.3746(2) 0.3390(2) 0.8223(1) 0.0320(7) 0.0296(7) 0.0272(7) 0.0002(6) -0.0088(5) -0.0093(6) C(3) 2 i 0.4090(1) 0.2542(1) 0.7455(1) 0.0286(7) 0.0273(7) 0.0273(7) 0.0011(5) -0.0083(5) -0.0088(6) C(4) 2i 0.3511(2) 0.1141(2) 0.8244(2) 0.0378(8) 0.0280(7) 0.0318(8) -0.0024(6) -0.0043(6) -0.0102(6) C(5) 2i 0.2694(2) 0.0678(2) 0.9564(2) 0.0427(9) 0.0327(8) 0.0333(8) -0.0063(7) -0.0048(6) -0.0080(6) C(6) 2i 0.2258(2) 0.1513(2) 1.0389(1) 0.0348(8) 0.0425(9) 0.0239(7) -0.0042(6) -0.0051(5) -0.0106(6) C(7) 2i 0.2732(2) 0.0762(2) 1.1787(2) 0.066(1) 0.050(1) 0.0314(9) -0.0119(9) -0.0184(8) -0.0027(8) C(8) 2i 0.0599(2) 0.1651(2) 1.0752(2) 0.0381(9) 0.065(1) 0.049(1) -0.0069(9) -0.0019(7) -0.028(1) C(9) 21 0.4927(1) 0.2997(1) 0.6112(1) 0.0273(7) 0.0238(7) 0.0263(7) 0.0014(5) -0.0079(5) -0.0093(5) C(10) 2i 0.5448(1) 0.4441(1) 0.5183(1) 0.0242(7) 0.0263(7) 0.0221(6) -0.0012(5) -0.0039(5) -0.0089(5) C ( l l ) 2i 0.7988(2) 0.3868(1) 0.5340(2) 0.0277(7) 0.0213(7) 0.0415(8) 0.0000(5) -0.0119(6) -0.0099(6) C(12) 21 0.7713(2) 0.3491(2) 0.6780(2) 0.0373(9) 0.0303(8) 0.0415(8) -0.0034(6) -0.0172(7) -0.0065(6) C(13) 2i 0.8772(2) 0.2834(2) 0.7506(2) 0.054(1) 0.0385(9) 0.058(1) -0.0098(8) -0.0335(9) -0.0017(8) C(14) 2i 1.0107(2) 0.2560(2) 0.6794(3) 0.047(1) 0.038(1) 0.094(2) 0.0025(8) -0.045(1) -0.010(1) C(15) 2i 1.0375(2) 0.2938(2) 0.5375(3) 0.0282(9) 0.039(1) 0.100(2) 0.0052(7) -0.0190(9) -0.030(1) C(16) 2 i 0.9345(2) 0.3595(2) 0.4604(2) 0.0256(7) 0.0320(8) 0.065(1) -0.0007(6) -0.0079(7) -0.0233(7) C(17) 2i 0.9654(2) 0.4014(2) 0.3044(2) 0.0331(9) 0.060(1) 0.071(1) -0.0038(8) 0.0059(8) -0.042(1) C(18) 21 0.5555(2) 0.2311(1) 0.4147(1) 0.0337(8) 0.0269(7) 0.0288(7) -0.0052(6) -0.0049(5) -0.0110(6) C(19) 2i 0.4574(2) 0.3217(2) 0.3439(2) 0.0369(8) 0.0328(8) 0.0406(8) -0.0023(6) -0.0120(6) -0.0165(7) C(20) 2i 0.4671(2) 0.3518(2) 0.2042(2) 0.055(1) 0.0413(9) 0.0471(9) -0.0016(8) -0.0260(8) -0.0185(8) C(21) 2f 0.5734(2) 0.2916(2) 0.1343(2) 0.064(1) 0.049(1) 0.0362(9) -0.0074(8) -0.0175(8) -0.0198(8) C(22) 2i 0.6676(2) 0.1975(2) 0.2069(2) 0.049(1) 0.0431(9) 0.0393(9) -0.0065(8) -0.0038(7) -0.0240(7) C(23) 2i 0.6607(2) 0.1641(2) 0.3466(2) 0.0368(8) 0.0282(7) 0.0337(8) -0.0035(6) -0.0039(6) -0.0140(6) C(24) 2i 0.7603(2) 0.0595(2) 0.4251(2) 0.047(1) 0.0393(9) 0.0388(9) 0.0093(8) -0.0055(7) -0.0178(8)
Acknowledgments. We thank the Petroleum Research Foundation, the Uni- versity of Utah, and the National Science Foundation for partial support of this work.
References
1. Basta, R.; Ernst, R. D.; Arif, A. M.: Synthesis and Structure of the Edge- Bridged Open Zirconocene, Zr(6,6-dmch>2(PMe3)2 (dmch = dimethyl- cyclohexadienyl), and its Imine Coupling Product. J. Organometal. Chem.
683 (2003) 64-69.
2. Basta, R.: Synthesis and Reactivity Patterns of Edge-Bridged Open and Half-open Metallocenes. Ph.D. Dissertation, University of Utah, Salt Lake City, USA 2004.
3. Harvey, B. G.; Arif, A. M.; Ernst, R. D.: Incorporation of Polybasic Aro- matic Amines Into Ruthenium(H) Chloro Complexes. Polyhedron 23 (2004) 2725-2731.
4. Altomare, A.; Burla, M. C.; Camalli, M.; Cascarano, G. L.; Giacovazzo, C.; Guagliardi, A.; Molitemi, A. G. G.; Polidori, G.; Spagna, R.: SIR97 - a new tool for crystal structure determination and refinement. J. Appl.
Crystallogr. 32 (1999) 115-119.
5. Sheldrick, G. M.: SHELXL-97. Program for the Refinement of Crystal Structures. University of Gottingen, Germany 1997.