Study of the mechanochemical process to crystalline Cu
2ZnSnS
4powder
Anna Ritscher
a,b, Marc Schlosser
c, Arno P fi tzner
c, Martin Lerch
a,*
aInstitutfürChemie,TechnischeUniversitätBerlin,Straßedes17.Juni135,10623Berlin,Germany
bHelmholtz-ZentrumBerlinfürMaterialienundEnergie,AbteilungKristallographie,Hahn-Meitner-Platz1,14109Berlin,Germany
cInstitutfürAnorganischeChemie,UniversitätRegensburg,Universitätsstr.31,93040Regensburg,Germany
ARTICLE INFO
Articlehistory:
Received17May2016
Receivedinrevisedform26July2016 Accepted5August2016
Availableonline6August2016
Keywords:
A.Semiconductors B.Chemicalsynthesis C.X-raydiffraction D.Crystalstructure D.Defects
ABSTRACT
Kesterite-typeCu2ZnSnS4wassynthesizedfromthecorrespondingbinarysulfidesbyamechanochemi- calrouteinaplanetaryballmill.Thereactionprogressduringthismillingstepwasfollowedwithinatime rangeof10–180minbypowderX-raydiffraction.Inaddition,thecrystallizationofthemilledmaterial wasstudiedinsitubyhigh-temperatureX-raydiffractionmethodsinthetemperaturerangeof300–
500C. Significantdisorder(cationdistribution)wasobservedat500C,stronglydecreasingduring coolingdowntoambienttemperaturewitharateof60K/h.
ã2016ElsevierLtd.Allrightsreserved.
1.Introduction
Systematic analysis of the semiconductor compound Cu2ZnSnS4 (CZTS) and its structural, chemical, and physical propertieshavebeeninthefocusofinterestinthelastfewyears [1–6].Thisquaternarychalcogenideexhibitsagreatpotentialas absorbermaterialforapplicationsinthinfilmphotovoltaics.Due to its suitable properties (optical band gap energy=1.5eV, absorption coefficient in the order of 104cm1 [7–9]) this materialis discussedasa promising low-cost alternativetoIn- containingCuInxGa(1-x)Se2(CIGS).Thecurrentrecordefficiencyof 12.6%wasreachedforCZTS-baseddevicescontainingadditional selenium[10].
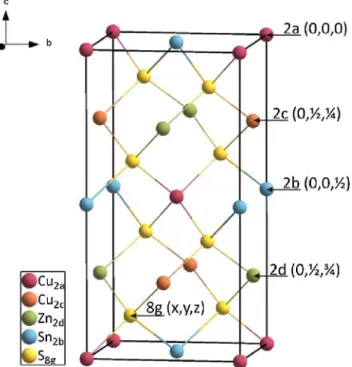
Aftersomecontroversiesaboutthecorrectcrystalstructureof Cu2ZnSnS4 it was demonstrated in different studies that this quaternary sulfide adopts the kesterite structure type (space groupI4) [11,12]. Thistetragonalcrystal structure derives from thesphaleritestructuretype(knownforZnSetc.)bydoublingthe c-axisanditconsistsofalternatingcationlayersstackedalongthis axis(seeFig.1).
Thelatticeplanesatz=0andz=1/2areoccupiedbyCuandSnin anorderedmanner,i.e.CuisfoundonWyckoffposition2a(0,0,0) andSnonposition2b(0,0,1/2).RemainingCuandZnatomsare
locatedonlatticeplanesatz=1/4andz=3/4,position2c(0,1/2,1/4) and 2d (0,1/2, 3/4), respectively. Ab initio calculations [13,14]
showedthat,duetoitsverylowformationenergy,thepointdefect complex(CuZn+ZnCu)iseasilyformedandthusCu/Zndisorderhas tobeexpected.Thiswasalsoconfirmedbydiffractionstudieson CZTSpowdersamples,wherepartial[15,16]orcompletedisorder [1]ofCuandZnonthe2cand2dpositionswasreported.
HightemperatureX-raydiffractionmeasurementsofkesterite powderusingsynchrotronradiationshowthatCu2ZnSnS4under- goesastructuralphasetransitionfromthetetragonalkesterite-to the cubic sphalerite-type structure at temperatures above of 866C [2]. Thetransitionleadstoa randomdistributionof the cationsCu,Zn,andSn(spacegroupF43m).Duetotheformationof secondary phases it is challenging to prepare phase-pure CZTS powder, whichis importantfordetailed studiesconcerningthe correlations betweenstructural and electronic properties. Bulk materialofCZTShasbeenusuallyproducedbysolidstatereaction oftheelementsinevacuatedsilicaampoules[1,17].Duetothehigh sulfur vapor pressure it is necessary to apply a well-defined temperatureprogramandhomogenizationisachievedbyasecond annealingstepat750C.Allthesefactorsresultinlongreaction timesandanenhancedpossibilityfortheformationofsecondary phases.
Recently we presented an easy and fast process for the preparation of single phase CZTS powders [16]. Themain idea was the development of a synthesis route at temperatures significantly lower than 750C. This was realized by a
*Correspondingauthorat:
E-mailaddress:martin.lerch@tu-berlin.de(M.Lerch).
http://dx.doi.org/10.1016/j.materresbull.2016.08.006 0025-5408/ã2016ElsevierLtd.Allrightsreserved.
ContentslistsavailableatScienceDirect
Materials Research Bulletin
j o u r n al h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / m a t r e s b u
mechanochemicalapproachandthesynthesisofaprecursorby reactionofthecorrespondingbinarysulfidesCuS,ZnS,andSnSina planetaryballmill.Mechanochemicalprocessesinplanetaryball millsfinda widespreadusein scienceand industrydue tothe feasibility of rapid, highly efficient and usually solvent-free chemical synthesis. Additionally, it is possible todevelop com- pounds that cannot be obtained via a conventional solid-state route[18].
After annealing at 500C in a H2S atmosphere, a highly crystalline product is obtained. A similar mechanochemical process was reported in Ref. [19], where Cu2ZnSnX4 (X=S, Se) bulkmaterialwaspreparedinaballmillstartingfromthemetals andthechalcogens.
Inthiscontributionwepresent amoredetailedstudyofour newtwo-stepsynthesisprocess.Thereactionprocessintheball millasfunctionoftimeaswellasthecrystallizationbehaviorofthe poorlycrystallineCZTSprecursortothecrystallinepowderwith temperatureareinvestigated.
2.Experimental
Sulfide powderswiththechemical formulaCu2ZnSnS4 were preparedbyourmechanicalprocess[16].CuS,ZnS,andSnSwere mixed in a molar ratio of 2:1:1 without any additional fluid mediumandfilledintoan80ml agatejar(including 5grinding ballswithadiameterof20mm).MillingwasperformedinaFritsch PlanetaryMonoMillPULVERISETTE6usingarotationalspeedof 400rpmandmillingtimesofupto180min.Smallamountsofthe sample were withdrawn at time intervals of 10–30min for diffractionanalyses.
Inordertogetahighly crystallineproductasreference,onehalfof theas-milledpowder (millingtime180min)was annealed in a conventional tube furnace equipped with a SiO2-tube in H2S- atmospherefor3hat500C.Afterthistreatment,thesamplewas cooleddown witharateof60K/h.X-raypowderdiffractionwasused forthestructuralcharacterizationofallsamples.Diffractiondata
werecollectedusingaPanalyticalX’PertPROdiffractometer(Bragg- Brentanogeometry,Cu-K
a
radiation).Structuralrefinementswere performedbytheRietveldmethod[20]withtheprogrampackage FullprofSuiteVersion2015[21]by applyingapseudo-Voigtfunction.Thekesterite-typestructure(spacegroupI4)wasusedasstarting modelfortherefinement.
Thesecondhalfofthesamplewasusedfortheinvestigationof thecrystallizationbehaviorofthequaternarysulfideprecursorby insituhigh-temperatureX-raydiffractionwiththehelpofaSTOE STADI Pdiffractometer(Transmission/Debye Scherrer geometry, Mo-K
a
1 radiation). The instrument was equipped witha STOE furnaceusingaNi/CrNithermocouple.Thesamplewasencapsu- latedinanevacuatedsilicacapillaryandinitiallymeasuredatroom temperature(RT,measurementnumber001).Thenthesamplewas heated to 300C with a rate of 50K/min and subsequently measuredat300Cevery10mininordertofollowtheisothermal phaseformationandgrowthofthecrystallites(20measurements, 1min, measurement numbers N=002–021). Afterwards, the temperature was raised to 400C and the powder was again measured every10min(measurementnumbersN=022–042).A furtherheatingstepuptoa temperatureof 500C wasapplied (measurement numbers N=043–062). Againdiffraction pattern were recorded every 10min. As a reference measurement a diffractionpatternoftheemptyfurnacewithoutanysamplewas recorded in order to detect the signals from the furnace (measurementnumberN=000).3.Resultsanddiscussion
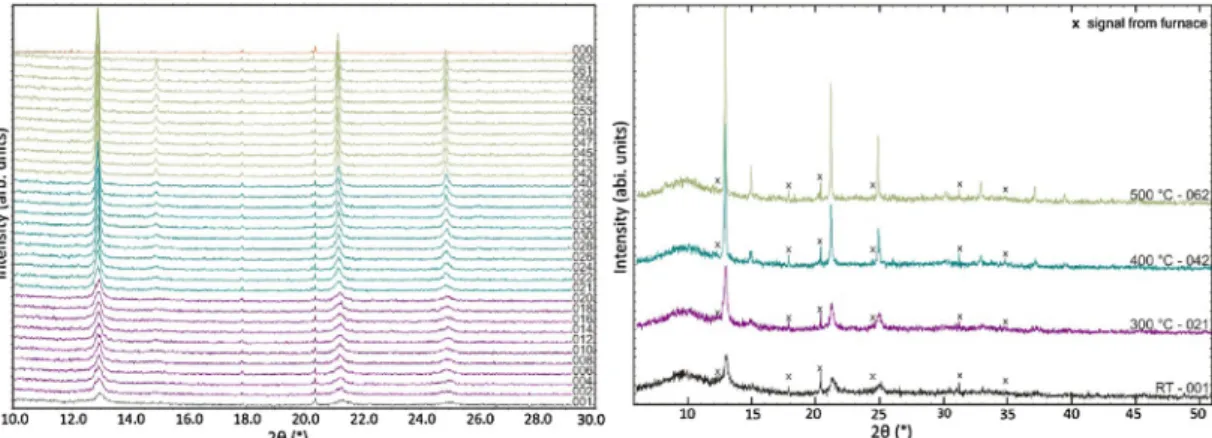
Asdescribed in theexperimental section, thefirstsynthesis step to phase pure Cu2ZnSnS4 powder is the reaction of the correspondingbinarysulfides(CuS,ZnS,SnS)inaballmill.Inorder tofollow this reactionprocess, smallamounts of powderwere withdrawn from the grindingbowl by interrupting themilling procedure several times.Fig.2 depictstheXRDpatterns ofthe preparedCZTSpowdersmilledfor10–180min.
Uptoamillingtimeof30minthereflectionsoriginatingfrom thebinarysulfidesarethemainsignalsinthediffractionpatterns.
Duringthisearlystageofmilling,thepowdersofCuS,ZnS,andSnS aremixedtogetherand noreactiontakesplace.After40minof millingtheintensitiesofthereflectionsthatcanbeassignedtothe Fig.1.Unitcelloffullyorderedkesterite-typeCu2ZnSnS4,alloccupiedWyckoff
positionsarelabeled.
Fig.2.Diffractionpatterns(Cu-Karadiation)ofas-preparedsamplesafterthe millingprocessatdifferenttimes(t=10–180min).
binarycompounds begin todecrease, which indicatesthat the solid-statereactionisactivatedandtheproductstartstoform,and after150minitisclearlyseenthatthesignalshavedisappeared.
The sample was further treated up to a total milling time of 180min. However, the diffraction pattern did not change significantly,thus atthis stagethereaction isconsideredtobe terminated.Takingalookatthechangeofthereflectionpositionof themajorreflection a cleartrend to lower2
u
values is visible(2
u
=28.68!28.62),which is anindicationthat thequaternary compound is formed during the reaction in the ball mill. The observed reflection positions of the final as-milled powder correspond to the strongest ones expected for the quaternary compound.However,fromtheobtainedX-raydiffractionpatterns itisnotpossibletoextractunequivocalinformationconcerningthe distributionofthecationsasthemainreflectionsofthekesterite phase (partially or fully ordered) practically have the same positions as the expected ones for a sphalerite-type structure (fully disordered) with the same chemical composition. The superlattice reflections for the kesterite-type phase are rather small(seeFig.4)buttheirtotalabsencepointstoseveredisorderin the milled material. For more unambiguous results additional investigations,forexamplebyEXAFS,wouldbenecessary.Inaddition,asitcanbeclearlyseeninFig.2themillingprocess resultsinverybroadreflections.TheFWHMvaluesincreasewith increasingmillingtimeasshowninFig.3.Profileanalysesrevealed adominantLorentzianshapewhichcanbeattributedtocrystallite sizebroadening.Thecrystallitesizesoftheprecursorpowdersare calculatedusing theScherrer equation (Eq.(1))fromthe strong 112-reflectionat2
u
28.6[22],D
ð2u
Þ¼ Kl
Lcos
u
0 ð1Þwhere
D
(2u
)is the line width at half the maximum intensity(FWHM),KistheScherrer-formfactor,inthisstudyafactorof1 wasused,
l
isthewavelength,Lthemeansizeofthecrystallite, andu
0thediffractionangle.D
(2u
)hastobecorrectedforreflex broadening comingfrom the instrument according toD
ð2u
Þ¼ ðb
2Mb
2IÞ1=2(b
MismeasuredFWHMandb
Iisthecorrectionfactorforinstrumentbroadening).
AsdepictedinFig.3,theparticlesizeafter10minofmillingis calculatedto37nm.Inthefirst90minthecrystallitesizedecreases rapidly,approachingavalueofabout15nm.Onfurthermillingthe crystallitesizeslightly changes reachinga sizeof 11nmafter 180min. In order to confirm the calculated value, TEM
measurementswerecarriedoutwiththissample.Theobserved crystallitesizeswerefoundtobeinarangeof8–13nmwhichisin goodagreementwiththeresultoftheScherrermethod(11nm).
Inordertoprepareahighlycrystallinereferencesample,one halfoftheprecursorpowderwasannealedinatubefurnace(H2S atmosphere)accordingtothesecondstepofourmechanochemical process[16]. Thestructuralparametersof theannealedsample wererefined(seeTables1and2).Asitwasshowninourrecent studies, it is nicely possible to control the composition of the synthesizedpowderwiththemechanochemicalmethod[16,23].
Therefore, for the structural refinement the ideal composition Cu2ZnSnS4wasused.TheX-raypowderdiagramwiththeresultsof theRietveldrefinementisdepictedinFig.4.
Itshouldbementionedthatitisnotpossibletodifferentiate betweenZnandCuusingconventionalX-raypowderdiffraction methods.Consequently,wecanonlydistinguishbetweenCu/Zn andSn.Unfortunately,theamountofpreparedmaterialwasnot sufficientforneutrondiffractionexperiments.Fortherefinements, thefollowingsideconditionswereset:thesumofCu/Znonall positionsmustbe3,thesumofSnonallpositionsmustbe1,andall fourcationpositionshavetobefullyoccupied.
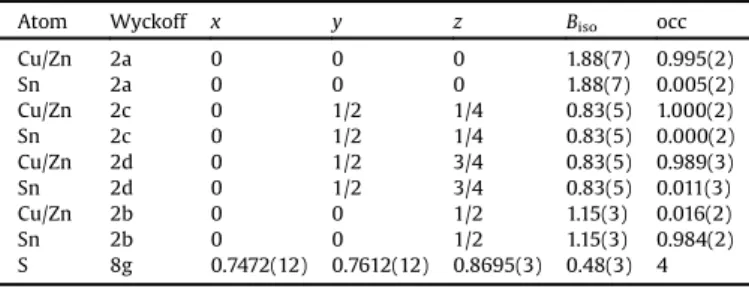
Table 2 lists the final atomic and additional structural parameters.Asexpected, Snwas foundonWyckoffposition2b (0,0,1/2),whereasCu/Znislocatedon2a(0,0,0),2c(0,1/2,1/4), and2d(0, 1/2,3/4)sites.NoSnisfoundonposition2c.On2aand2d sitesverysmallamountsofSn(around1%)arefound.MissingSn onthe2bsiteisreplacedbyCu/Zn.Theseresultsareinagreement withourrecentrefinements[16].
Fig.4. X-raypowderdiffractionpatternwiththeresultsoftheRietveldrefinement usingthekesterite-typestructureasstartingmodel.
Fig.3.FWHMvaluesandcorrespondingcrystallitesizesduringreactionintheball mill.
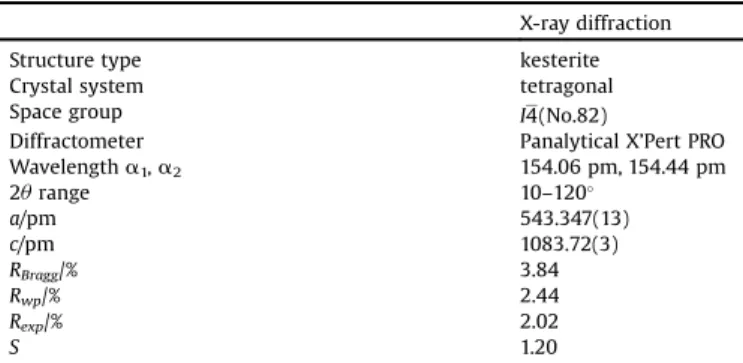
Table1
LatticeparametersofCZTSandresidualvaluesoftheRietveldrefinement.
X-raydiffraction
Structuretype kesterite
Crystalsystem tetragonal
Spacegroup I4(No.82)
Diffractometer PanalyticalX’PertPRO
Wavelengtha1,a2 154.06pm,154.44pm
2urange 10–120
a/pm 543.347(13)
c/pm 1083.72(3)
RBragg/% 3.84
Rwp/% 2.44
Rexp/% 2.02
S 1.20
Thesecondhalfoftheas-milledprecursorwasusedtoexamine theeffectof heattreatmentonthecrystallization of mechano- chemicallysynthesizedCZTSpowderbyinsituhigh-temperature X-raydiffraction.Aspointedoutintheexperimentalsection,some seriesofisothermalmeasurementsat300,400,and500Cwere performed.The evolution of theXRD patternscollectedduring heating is depictedin Fig.5 and 6. For a firstoverview, Fig. 5 presents a 3D plot and a rainbow contour plot of all high temperatureX-raypatterns.
Inordertoavoidinformationaloverflow,Fig.6(left)presents onlyonehalf ofmeasuredpatterns(including thepatternwith signalsfromtheemptyfurnace),whereasinFig.6(right)thelastin situ measurement of each annealing temperature (300, 400, 500C)aswellasthediffractionpatternat25C isshown.Isit clearlyseenthatthereflectionsofas-milledCZTSbecamesharper withheatingtime,indicatingincreasedcrystallinity.
Asexpected,increasingtemperatureledtoadecreaseofthefull width at half maximum (FWHM) values (see Fig. 7a). The measurementof thestarting material(25C) givesa calculated crystallitesizeof12nm,whichisingoodagreementwiththeresult fromthediffractometerworkingwithCu-K
a
radiation(SeeFig.4).Having a closer look at the results coming from different annealing temperatures significant differences are found (See Fig.7b).At300Cthecrystallizationisratherslow,thecrystallite sizesbarelyincrease.After3.5hsizesof15nmarereached(see Fig.7b).Asexpected,asignificantlyfasterincreaseisobservedfor 400C, where a final size of 37nm is calculated. In the last annealingstepat500Cthecrystallitesizeisstillincreasingand reachesavalueof50nmafter3.5h.
AsshowninFig.7b,thecrystallitesizeincreasesalmostlinearly at400Candabove.
Usingthefinaldatasetmeasuredat500C(N=062),thecrystal structure was also refined. The X-ray powder patternwith the resultsof the Rietveld refinement is depictedin Fig. 8. Lattice
parameters and residualvalues are summarized in Table 3. As alreadydescribedabove,spacegroupI4 wasusedtogetherwith additional sideconditions ascopperand zinc cannotbedistin- guishedbyconventionalX-raydiffractionmethods.Table4 lists thefinalatomicandotherstructuralparameters.
SnismainlylocatedonWyckoffposition2b,whereasCu/Znis foundontheotherthreecationsites.NosignificantamountofSn wasfoundonpositions2aand2c.Incontrast,asignificantamount of tin (8%)was foundon the 2dsite, whereas missing Sn is replacedbyCu/Znon2b.ForCZTSitisknownthatintrinsicdefects can beeasily formedwhile the kesterite–type structure (space groupI4)ismaintained,e.g.[17,24–26].Theoreticalwork[14,27]
hasshownthatthedefectcluster[Zn2Sn+Sn2+Zn]hasrelatively lowformationenergy(around0.9eV/pair).ThereforeSnCu/Znand Cu/ZnSndefectsshouldbepresentwhenincreasingthetempera- ture.KeepinmindthatZnandCucannotbedistinguishedwith conventional X-ray diffraction techniques. Consequently, the actualdefectclustersinthepresentsamplecannotbedetermined unequivocally.
The refined valuescanbecompared withthevalues forthe above-presentedsampleannealedat500Candcooleddownwith arateof60K/h.At500C,SnCu/ZnandCu/ZnSnanti-sitedefectsare clearlypresent;inthesampleslowlycooleddownto25Chardly anySndisorderisfound.Consequently,itcanbepresumedthat these defectsheal outrapidly during coolingdowntoambient temperature.Thisisalsocomparabletoourrecentneutronpowder diffractionstudyonsamplesannealedforweeks[23]:upto350C no significant disorder of Sn on the remaining three cation positions(2a,2c,2d)isoccurring.
Itcanbeexpectedthattheamountofpointdefectsisincreasing with increasing temperature, finally resulting in a statistical distributionofcopper,zinc,andtin.Asalreadymentioned,sucha high-temperature order-disorder transition was observed by Schorr and Gonzales-Aviles [2],resulting in a cubic sphalerite- typecrystalstructure.
4.Conclusion
Inthisworkourrecentlydevelopedmechanochemicalsynthe- sisprocesstophasepureCZTSpowderwasinvestigatedinmore detail. The reaction of the binary sulfides in the ball millwas followedbyX-raydiffractionmeasurements.Thecrystallitesizeof theas-milledCZTSpowderapproachesavalueof10nmafter3hof milling.Inaddition,thecrystallizationbehaviorwithtemperature wasinvestigatedbyinsituhigh-temperatureX-raydiffraction.At 500C,SnCu/ZnandCu/ZnSnanti-sitedefectsareclearlyobserved.
Their concentrationstronglydecreases when coolingdownthe Table2
RefinedstructuralparametersforCZTSfromX-raydiffraction.
Atom Wyckoff x y z Biso occ
Cu/Zn 2a 0 0 0 1.88(7) 0.995(2)
Sn 2a 0 0 0 1.88(7) 0.005(2)
Cu/Zn 2c 0 1/2 1/4 0.83(5) 1.000(2)
Sn 2c 0 1/2 1/4 0.83(5) 0.000(2)
Cu/Zn 2d 0 1/2 3/4 0.83(5) 0.989(3)
Sn 2d 0 1/2 3/4 0.83(5) 0.011(3)
Cu/Zn 2b 0 0 1/2 1.15(3) 0.016(2)
Sn 2b 0 0 1/2 1.15(3) 0.984(2)
S 8g 0.7472(12) 0.7612(12) 0.8695(3) 0.48(3) 4
Fig.5.3Dplot(left)andrainbowcontourplot(right)forin-situheattreatmentofCZTSprecursorpowderupto500C(Mo-Ka1radiation).Intherainbowcolorcontourplot intensitiesareshownascolorscalewherelowcountsareblue,whilehigherintensitiesareshownasredandyellow.Temperatureincreasesfromthebottomtothetop.(For interpretationofthereferencestocolourinthisfigurelegend,thereaderisreferredtothewebversionofthisarticle.)
Fig.6.EvolutionofthepowderpatternsprobedbyinsituhightemperatureX-raydiffraction(Mo-Ka1radiation).Thenumberofeachpatterncorrespondstothe measurementnumberN(seeexperimentalsection).Pink:300C,blue:400C,green:500C.Thestartingpatternat25Cisshowninblack,thepatternoftheemptyfurnace isdepictedinorange.(Forinterpretationofthereferencestocolourinthisfigurelegend,thereaderisreferredtothewebversionofthisarticle.)
Fig.7.FWHMvalues(a)andcalculatedcrystalsizes(b)fortheCZTSprecursor.
Fig.8. Hightemperature(500C)X-raypowderdiffractionpatternwiththeresultsoftheRietveldrefinement(excludedregions=signalscomingfromthefurnace).
sample.Thecoolingrateof60K/hseemstobethelimit forthe production ofsamples withlow Sn-involveddisorder, which is importantfortheperformanceofCZTSinsolarcells.Foramore detailedanalysisoforderingprocessesuptothedisorderedcubic phase, in situ high-temperature neutron powder diffraction investigationsareplanned.
Acknowledgements
Financial support from the MatSEC graduate school of the HelmholtzZentrumBerlin(HZB)incooperationwiththeDahlem
Research School is gratefully acknowledged. Special thanks to Martin Rohloff (AG Anna Fischer) for performing the TEM measurements.
References
[1]S.Schorr,H.-J.Hoebler,M.Tovar,Eur.J.Mineral.19(2007)65–73.
[2]S.Schorr,G.Gonzalez-Aviles,Phys.StatusSolidiA206(2009)1054–1058.
[3]J.Just,D.Luetzenkirchen-Hecht,R.Frahm,S.Schorr,T.Unold,Appl.Phys.Lett 99(2011)262105/1–262105/3.
[4]S.Chen,X.G.Gong,A.Walsh,S.-H.Wei,Appl.Phys.Lett.96(2010)021902/1–
021902/3.
[5]S.Chen,L.-W.Wang,A.Walsh,X.G.Gong,S.-H.Wei,Appl.Phys.Lett.101(2012) 223901/1–223901/4.
[6]A.J.Jackson,A.Walsh,J.Mater.Chem.A(2014)7829–7836.
[7]S.Chen,X.G.Gong,A.Walsh,S.-H.Wei,Appl.Phys.Lett.94(2009)041903/1–
041903/3.
[8]J.J.Scragg,P.J.Dale,L.M.Peter,G.Zoppi,I.Forbes,Phys.StatusSolidiB245 (2008)1772–1778.
[9]S.Siebentritt,S.Schorr,Prog.Photovolt.20(2012)512–519.
[10]W.Wang,M.T.Winkler,O.Gunawan,T.Gokmen,T.K.Todorov,Y.Zhu,D.B.
Mitzi,Adv.EnergyMater4(2014)1301465/1–1301465/5.
[11]S.Schorr,Sol.EnergyMater.Sol.Cells95(2011)1482–1488.
[12]L.Choubrac,M.Paris,A.Lafond,C.Guillot-Deudon,X.Rocquefelte,S.Jobic, Phys.Chem.Chem.Phys.15(2013)10722–10725.
[13]A.Nagoya,R.Asahi,R.Wahl,G.Kresse,Phys.Rev.BCondens.MatterMater.
Phys81(2010)113202/1–113202/4.
[14]S.Chen,J.-H.Yang,X.G.Gong,A.Walsh,S.-H.Wei,Phys.Rev.B:Condens.Matter Mater.Phys81(2010)245204/1–245204/10.
[15]S.Schorr,M.Tovar,BENSCExperimentalReport,(2006).
[16]A.Ritscher,J.Just,O.Dolotko,S.Schorr,M.Lerch,J.AlloysCompd.670(2016) 289–296.
[17]L.E.ValleRios,K.Neldner,G.Gurieva,S.Schorr,J.AlloysCompd.657(2016) 408–413.
[18]C.F.Burmeister,A.Kwade,Chem.Soc.Rev.42(2013)7660–7667.
[19]D.Pareek,K.R.Balasubramaniam,P.Sharma,Mater.Charact.103(2015)42–49.
[20]H.M.Rietveld,J.Appl.Crystallogr.2(1969)65–71.
[21]J.Rodriguez-Carvajal,AbstractsoftheSatelliteMeetingonPowderDiffraction oftheXV,CongressoftheIUCr,1990,pp.127.
[22]P. Scherrer, Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen,Mathematisch-PhysikalischeKlasse1918(1918).
[23]A.Ritscher,M.Hoelzel,M.Lerch,J.SolidStateChem.238(2016)68–73.
[24]G.Gurieva,M.Dimitrievska,S.Zander,A.Perez-Rodriguez,V.Izquierdo-Roca, S.Schorr,Phys.StatusSolidiC(2015)1–4.
[25]A.Lafond,L.Choubrac,C.Guillot-Deudon,P.Deniard,S.Jobic,Z.Anorg.Allg.
Chem.638(2012)2571–2577.
[26]L.Choubrac,A.Lafond,C.Guillot-Deudon,Y.Moelo,S.Jobic,Inorg.Chem.51 (2012)3346–3348.
[27]S.Chen,A.Walsh,X.-G.Gong,S.-H.Wei,Adv.Mater.(Weinheim,Ger.)25(2013) 1522–1539.
Table3
LatticeparametersandresidualvaluesoftheRietveldrefinementofCZTSat500C.
X-raydiffraction
Structuretype kesterite
Crystalsystem tetragonal
Spacegroup I4(No.82)
Diffractometer STOESTADIP
Wavelength 70.931pm
2urange 10–50
T(Measurement) 500C
a/pm 546.28(3)
c/pm 1090.10(8)
RBragg/% 4.77
Rwp/% 3.84
Rexp/% 3.49
S 1.10
Table4
RefinedstructuralparametersforCZTSat500CfromhightemperatureX-ray diffractiondata.
Atom Wyckoff x y z Biso occ
Cu/Zn 2a 0 0 0 4.00(18) 0.997(11)
Sn 2a 0 0 0 4.00(18) 0.004(11)
Cu/Zn 2c 0 1/2 1/4 4.32(13) 1.000(2)
Sn 2c 0 1/2 1/4 4.32(13) 0.000(2)
Cu/Zn 2d 0 1/2 3/4 4.32(13) 0.922(16)
Sn 2d 0 1/2 3/4 4.32(13) 0.080(16)
Cu/Zn 2b 0 0 1/2 2.36(8) 0.084(11)
Sn 2b 0 0 1/2 2.36(8) 0.918(11)
S 8g 0.748(6) 0.757(5) 0.8699(10) 1.94(9) 4