Synthesis and Coordination Chemistry of Anionic Pnictogenylborane Derivatives
Volltext
Abbildung
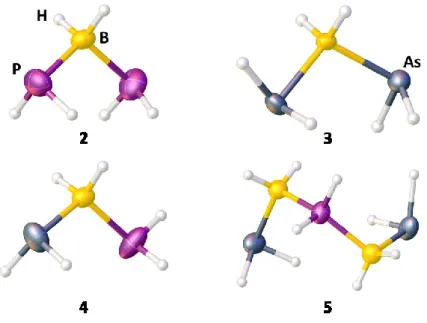
![Figure S 3.2. Molecular structure of 3 in the solid state. Selected bond lengths [Å] and angles [°]: As-B:](https://thumb-eu.123doks.com/thumbv2/1library_info/3737445.1509093/47.892.159.735.363.741/figure-molecular-structure-solid-state-selected-lengths-angles.webp)
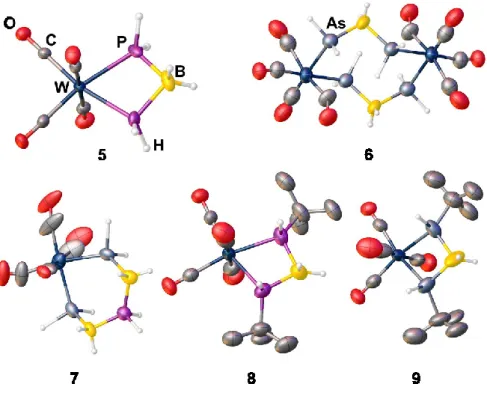
![Figure S 3.5. Molecular structure of the anion of 6 in the solid state. Selected bond lengths [Å] and angles[°]:](https://thumb-eu.123doks.com/thumbv2/1library_info/3737445.1509093/50.892.283.617.301.695/figure-molecular-structure-anion-solid-selected-lengths-angles.webp)
ÄHNLICHE DOKUMENTE
In order to get the 3-chloropropyl-substituted o- carborane derivatives in a one-step reaction starting from o-carborane, we tried the reaction of dilithio- o-carborane (formed in
Kantlehner and coworkers (Stuttgart/Aalen) give an account of their studies on the reaction of N, N, N , N - tetramethyl-chloroformamidinium chloride with sodium,
After N-galactosylation and subsequent O-sil- ylation, nucleophilic addition of organometallic reagents proceeded with high regio- and stereo- selectivity at 4-position. Substituents
The 1 : 1 iminium intermediate, generated by the addition of a secondary amine to acetalde- hyde is trapped by the (N-isocyanimino)triphenylphosphorane in the presence of
b Chemistry Department, Faculty of Science, Menoufi a University, Shebin El-Koam, Egypt.
The antimicrobial activity of the synthesized compounds was evaluated against three micro- organisms; Bacillus subtilis (ATCC 6633) (Gram- positive), Pseudomonas aeruginosa
Compared to an identical library generated by conventional parallel synthesis, a microwave- assisted procedure dramatically decreased reaction times from hours to minutes, and yields
The use of base required in such reactions was obviated by performing the re- action in water which not only avoided the use of base but also gave good yields (75 – 90 %) within 4.0
![Figure S 3.6. Molecular structure of the cation of 6 in the solid state. Selected bond lengths [Å] and angles [°]:](https://thumb-eu.123doks.com/thumbv2/1library_info/3737445.1509093/51.892.253.641.111.498/figure-molecular-structure-cation-solid-selected-lengths-angles.webp)
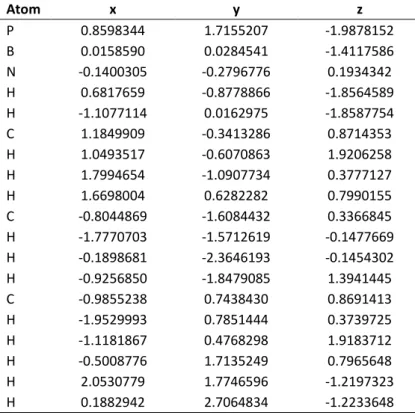
![Table S 3.8. Cartesian coordinates of the optimized geometry of [H 2 P-BH 2 -AsH 2 ] - at the B3LYP/def2-TZVP level of theory](https://thumb-eu.123doks.com/thumbv2/1library_info/3737445.1509093/64.892.111.789.377.766/table-cartesian-coordinates-optimized-geometry-tzvp-level-theory.webp)
![Table S 3.12. Cartesian coordinates of the optimized geometry of [H 2 As-BH 2 -AsH 2 -BH 2 -PH 2 ] - at the B3LYP/def2- B3LYP/def2-TZVP level of theory](https://thumb-eu.123doks.com/thumbv2/1library_info/3737445.1509093/66.892.117.529.235.566/table-cartesian-coordinates-optimized-geometry-tzvp-level-theory.webp)
![Table S 3.15. Cartesian coordinates of the optimized geometry of [PH 2 ] - at the B3LYP/def2-TZVP level of theory](https://thumb-eu.123doks.com/thumbv2/1library_info/3737445.1509093/68.892.109.787.803.1065/table-cartesian-coordinates-optimized-geometry-tzvp-level-theory.webp)