Ζ. Kristallogr. N C S 2 1 6 ( 2 0 0 1 )
19
© by O l d e n b o u r g W i s s e n s c h a f t s v e r l a g , M ü n c h e n
Crystal structure of trisodium holmium(III) hexachloride, Na^HoCle
M. Böcker, Ν. Gerlitzki and G. M e y e r *
Universität zu Köln. Institut für Anorganische Chemie. Greinstr. 6. D-50939 Köln. Germany
Received July 14. 2000, CSD-No. 409508
Abstract
Cl6HoNa
3, monoclinic,
P\2\ln\(No. 14),
a= 6.8683(9) Ä, b = 7.274( 1) Ä, c = 10.167( 1) Ä, β = 90.79(2)°, V = 507.9 Ä
3, Ζ = 2,
RRt(F) =0.017,
wRKf(F2)= 0.044, 7 = 293 K.
Source of material
Single crystals of Na3HoCl6 were obtained during numerous attempts to reduce H0CI3 with sodium metal (HoCb:Na = 1 : 1 ; tantalum container, 873 K) [1,2],
Discussion
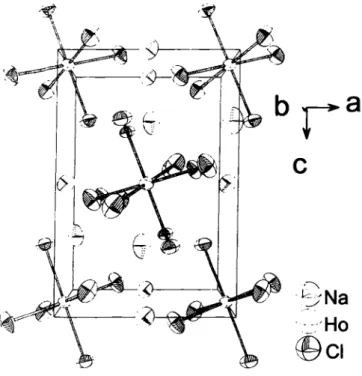
Na3HoCl6 crystallizes with the cryolite type of structure (Na3AlF6 [3]). Isotypic are a number of analogous ternary chlo- rides, Na
3MCl6 (M = Dy-Lu, Y, Sc) [4],
Table 1. Data collection and handling.
Crystal:
Wavelength:
μ:
Diffractometer, scan mode:
26niai:
WM/JmamiKd. NfAWjunique:
Criterion f o r /obs, N(hkl)tt: N(param)nf\vxA\
Programs:
transparent brown, irTegular, size 0.05 x 0.05 x 0.1 mm Mo Ka radiation (0.71073 A) 47.09 cm"1
Stoe-IPDS, φ 48°
2801,762 /obs > 2 o(/obsA 688 49
SHELXS-97 [5], SHELXL-97 [6], DIAMOND [7]
References
1. Böcker. Μ.: Synthese, Struktur, magnetische und spektroskopische Eigenschaften ternärer Halogenide des Vanadiums und Chroms mit Alkalimetallen und einiger Lanthanid-Analoga. Dissertation, Universität Hannover, Germany 1996.
2. Gerlitzki, N.: Unpublished results, Universität zu Köln, 2000.
3. Hawthorne, F. C.; Ferguson, R. B.: Refinement of the crystal structure of cryolite. Can. Min. 13 (1975) 377-382.
4. Meyer, G.; Ax, P.; Schleid, Th.; Irmler, M.: The Chlorides Na3MCl6 (M = Eu-Lu, Y, Sc): Synthesis, Crystal Structures and Thermal Behaviour. Z.
Anorg. Allg. Chem. 554 (1987) 25-33.
5. Sheldrick, G. M.: SHELXS-97-2, Program for the Solution of Crystal Structures. University of Göttingen, Germany 1997.
6. Sheldrick, G. M.: SHELXL-97-2, Program for Crystal Structure Refine- ment. University of Göttingen, Germany 1997.
7. Brandenburg, K.: Diamond (Version 2.1 a). Crystal Impact GbR, Germany 1996-1999.
T a b l e 2. Atomic coordinates and displacement parameters (in Ä2).
Atom Site X y ζ t / n U22 i/33 i/12 Un U23
H o ( l ) 2c 0 1/2 0 0.0188(2) 0.0149(2) 0.0159(2) -0.00060(8) 0.0005(1) -0.00160(8)
Cl(l) 4e 0.1383(2) 0.5648(2) 0.23939(9) 0.0337(6) 0.0318(5) 0.0182(4) 0.0026(5) -0.0040(4) -0.0032(5) Cl(2) Ae • 0.1679(2) 0.8052(1) -0.0773(1) 0.0356(6) 0.0260(5) 0.0461(6) -0.0130(4) -0.0082(5) 0.0122(5) Cl(3) 4e 0.3145(2) 0.3209(1) -0.0637(1) 0.0321(7) 0.0313(5) 0.0448(6) 0.0157(4) 0.0121(5) 0.0102(5)
N a ( l ) 2d 0 1/2 1/2 0.034(2) 0.026(1) 0.037(1) -0.0037(8) -0.006(1) 0.0028(9)
Na(2) 4e -0.0236(3) 0.9214(2) 0.2600(2) 0.034(1) 0.0261(9) 0.064(1) 0.0078(8) 0.0022(9) -0.007(1)
* Correspondence author (e-mail: g e r d . m e y e r @ u n i - k o e l n . d e )