Ζ. Kristallogr. NCS 217 (2002) 4 8 7 ^ 8 8
© by Oldenbourg Wissenschaftsverlag, München
487
Crystal structure of the low-temperature modification of trisodium heptaarsenide, LT-NajAs?
W. Hönle*·
1, J. Buresch", K. Peters
0, J. H. Chang" and H. G. von Schnering"
' Max-Planck-Institut für Chemische Physik fester Stoffe, Nöthnitzer Str. 40, D-01187 Dresden, Germany
" Max-Planck-Institut fur Festkörperforschung, Heisenbergstr. 1, D-70569 Stuttgart, Germany
Received September 20, 2002, accepted and available on-line November 5, 2002; CSD-No. 409658
As21 IAS23
As33 As32 Abstract
As7Na3, monoclinic, P12i/cl (No. 14), a = 15.554(2) Â,
b = 10.898(7) Â, c = 14.280(1) Â ,β = 115.83(1)°, V = 2178.8 Â3, Ζ = 8, Rp(F) = 0.070, wRreffF
2) = 0.145, T= 293 K.
Source of material
LT-Na3As7 is formed by reaction of stoichiometric mixtures of the elements in welded Nb-tubes (heating up to 773 Κ within 12 h;
reaction time at 773 K, 36 h; cooling to 633 Κ within 12 h;
annealing at 633 Κ for 48 h; cooling down to RT 24 h) [1-3]. The red brown colour of Na3As7 corresponds with the optical band gap of 2.3 eV (diffuse reflectivity; powder). Na3As7 is very sensitive to air and moisture.
Discussion
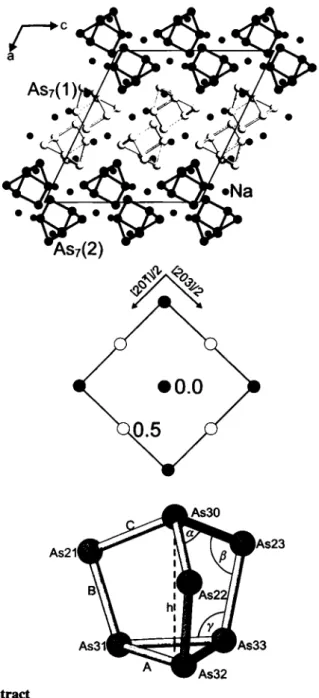
LT-Na3As7 forms a new structure type with two crystallographi- cally independent Zintl anions (As?)
3-. The arrangement of the cluster anions (upper figure) shows already the strong relationship to the plastically crystalline cubic Η T-modifìcation (LT / HT transition at 647 Κ [ 1,3]) representing a hierarchical cluster-replacement struc- ture of the cubic
LÌ3B1type [1,4-6]. A projection of the quasi- cubic cell of LT-Na3As7 with the equivalent unit-cell vectors is shown in the middle figure. The mean As—As bond lengths and bond angles (A=2.493 Â; Β = 2 . 3 6 2 Â; C = 2 . 4 1 0 Â ,
a=
100.5°;β= 99.6°, γ = 105.0° and Q = h/A = 3.358 / 2.493 = 1.35 (see lower figure) show relations which are characteristic for ionic nortricyclenes [5,7]. The Na
+cations connect four (As?)
3" anions
(d(Na—As = 2.95 Â - 3.41 Â), but show also the layout of typicalNaîAs7 units [1,5].
Table 1. Data collection and handling.
Crystal:
Wavelength:
ft-
Diffractometer, scan mode:
20max:
N(hkl)measured, hid)umquc'·
Criterion for /obs, N(ltklfo:
N(param)Kfmta\
Programs:
red brown fragment, size 0.20 χ 0.30 χ 0.30 mm Mo Ka radiation (0.71073 Â) 212.56 cm:1
Siemens P I , ω 55.04°
5181,5011 /obs > 2 a(Iobs), 2547 181
SHELXS-97 [9], SHELXL-97 [10]
ATOMS [11]
* Correspondence author (e-mail: hoenle@cpfs.mpg.de)
488
LT-Na3As7Table 2. Atomic coordinates and displacement parameters (in Â2).
Atom Site X y ζ Un t/22 ί/33 Un ί/13 ί/23
As(301) " 4e 0.5721(1) 0.3517(2) -0.1337(1) 0.036(1) 0.0267(9) 0.0320(8) 0.0031(7) 0.0139(7) 0.0067(7) As(311) 4e 0.6884(1) 0.0646(2) -0.0168(1) 0.042(1) 0.0318(9) 0.0375(9) 0.0117(8) 0.0205(8) 0.0064(7) As(321) 4e 0.7560(1) 0.2361(2) 0.1118(1) 0.0283(9) 0.049(1) 0.0259(7) 0.0014(8) 0.0092(7) 0.0018(8) As(331) 4e 0.5983(1) 0.1440(2) 0.0775(1) 0.040(1) 0.0332(9) 0.0351(9) 0.0001(8) 0.0242(8) 0.0068(7) As(211) 4e 0.6215(1) 0.1620(2) -0.1803(1) 0.047(1) 0.033(1) 0.0268(8) -0.0024(8) 0.0201(8) -0.0055(7) As(221) 4e 0.7174(1) 0.4176(2) 0.0110(1) 0.039(1) 0.036(1) 0.0412(9) -0.0145(8) 0.0240(8) -0.0074(8) As(231) 4e 0.4840(1) 0.2790(2) -0.0408(1) 0.0251(9) 0.0314(9) 0.052(1) 0.0033(7) 0.0194(8) 0.0041(8) As(302) 4e 0.9424(1) -0.3438(2) -0.2067(1) 0.041(1) 0.0280(9) 0.0360(8) 0.0106(8) 0.0208(8) 0.0005(7) As(312) 4e 0.8080(1) -0.0692(2) -0.2053(1) 0.056(1) 0.033(1) 0.0346(9) 0.0205(9) 0.0193(9) 0.0064(8) As(322) 4e 0.7427(1) -0.2514(2) -0.1506(1) 0.0278(9) 0.061(1) 0.0421(9) 0.0020(9) 0.0181(8) 0.0055(9) As(332) 4e 0.8931(1) -0.1511(2) -0.0259(1) 0.044(1) 0.0348(9) 0.0252(8) 0.0025(8) 0.0154(8) -0.0055(7) As(212) 4e 0.8874(1) -0.1545(2) -0.2979(1) 0.056(1) 0.033(1) 0.0296(8) 0.0003(9) 0.0237(8) 0.0060(7) As(222) 4e 0.7945(1) -0.4234(2) -0.2143(1) 0.041(1) 0.035(1) 0.0330(9) -0.0135(8) 0.0079(8) -0.0015(8) As(232) Ae 1.0182(1) -0.2708(2) -0.0293(1) 0.0290(9) 0.0324(9) 0.0317(8) 0.0011(7) 0.0072(7) 0.0031(7) Na(l) 4e 0.6013(5) 0.0361(6) -0.3715(5) 0.057(5) 0.040(4) 0.037(3) -0.006(3) 0.027(3) -0.007(3) Na(2) 4e 0.8975(5) -0.0403(6) -0.4787(5) 0.045(4) 0.036(4) 0.037(3) 0.007(3) 0.025(3) 0.004(3) Na(3) 4e 0.5622(5) -0.0620(7) 0.1991(5) 0.044(4) 0.042(4) 0.055(4) -0.007(3) 0.017(4) -0.001(3) Na(4) 4e 1.0698(6) -0.0560(7) -0.1330(5) 0.072(5) 0.039(4) 0.053(4) -0.019(4) 0.035(4) -0.007(3) Na(5) 4e 0.7926(5) 0.2963(7) -0.1529(5) 0.037(4) 0.065(5) 0.037(3) -0.010(4) 0.007(3) -0.009(3) Na(6) 4e 0.7129(5) -0.2084(8) 0.0648(5) 0.040(4) 0.077(6) 0.043(4) -0.006(4) 0.013(3) 0.006(4)
a: labelling of the As atoms As(yi) with i = homoatomic connectivity, j = position in cluster (lower figure), k = number of As7 cluster (upper figure).
References
1. Hönle, W.; von Schnering, H. G., Somer, M.: Strukturen von Hepta- arseniden der Alkalimetalle und deren Solvaten. Ζ. Kristallogr. 174 (1986) 82-83.
2. Hönle, W.: Über niedere Phosphide, Arsenide und Antimonide der Alkalimetalle. Dissertation, Universität Münster, 1975.
2. Buresch, J.: Binäre und temäre Arsenide, Antimonide und Arsenid- Antimonide der Alkalimetalle mit den Anionen X3", X35", X4 5", I[X~|, X71" und X75". Dissertation, Universität Stuttgart, 1996.
4. Meyer, Τ. M. : Verbindungen von Kalium, Rubidium und Cäsium mit den Polyanione Pt~, P73", P11 , AS73- und Asn3^. Dissertation, Universität Stuttgart, 1985.
5. von Schnering, H. G.; Hönle, W.: Bridging Chasms with Polyphosphides, Chem. Rev. 88 (1988) 243-273.
6. Carrillo-Cabrera, W.; Caroca-Canales, Ν.; von Schnering, H. G.:
K2i-¿Na2+<5ln39 (<5 = 2.8): A cluster-replacement Clathrate-D-Structure with an Alkali Metal Mi36-Network. Ζ. Anorg. Allg. Chem. 620 (1994) 247-257.
7. Hönle, W.; von Schnering, H. G.: Die Strukturen der Hepta-Hetero- Nortricyclene P7(Sime3)3 und P4(Sime2)4. Z. Anorg. Allg. Chem. 440 (1978) 171-182.
8. Sheldrick, G. M.: Phase Annealing in SHELX-90: Direct Methods for Larger Structures. Acta Crystallogr. A46 (1990) 467-473.
9. Sheldrick, G. M.: SHELXL-97. Program for the Refinement of Crystal Structures. University of Göttingen, Germany 1997.
10. Dowty, E.: ATOMS 5.1. A Complete Program for Displaying Atomic Structures. By Shape software, 521 Hidden Valley Road, Kingsport, TN 37663, USA 2000.